Related Research Articles
The actinide or actinoid series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium. The actinide series derives its name from the first element in the series, actinium. The informal chemical symbol An is used in general discussions of actinide chemistry to refer to any actinide.
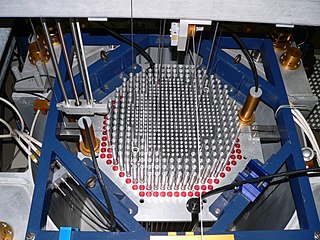
A nuclear reactor, formerly known as an atomic pile, is a device used to initiate and control a fission nuclear chain reaction or nuclear fusion reactions. Nuclear reactors are used at nuclear power plants for electricity generation and in nuclear marine propulsion. Heat from nuclear fission is passed to a working fluid, which in turn runs through steam turbines. These either drive a ship's propellers or turn electrical generators' shafts. Nuclear generated steam in principle can be used for industrial process heat or for district heating. Some reactors are used to produce isotopes for medical and industrial use, or for production of weapons-grade plutonium. As of early 2019, the IAEA reports there are 454 nuclear power reactors and 226 nuclear research reactors in operation around the world.

Radioactive waste is a type of hazardous waste that contains radioactive material. Radioactive waste is a result of many activities, including nuclear medicine, nuclear research, nuclear power generation, rare-earth mining, and nuclear weapons reprocessing. The storage and disposal of radioactive waste is regulated by government agencies in order to protect human health and the environment.

In nuclear engineering, fissile material is material capable of sustaining a nuclear fission chain reaction. By definition, fissile material can sustain a chain reaction with neutrons of thermal energy. The predominant neutron energy may be typified by either slow neutrons or fast neutrons. Fissile material can be used to fuel thermal-neutron reactors, fast-neutron reactors and nuclear explosives.
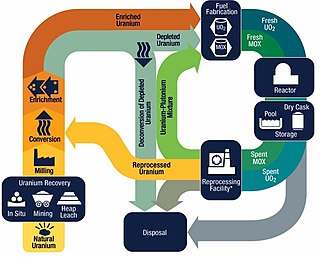
The nuclear fuel cycle, also called nuclear fuel chain, is the progression of nuclear fuel through a series of differing stages. It consists of steps in the front end, which are the preparation of the fuel, steps in the service period in which the fuel is used during reactor operation, and steps in the back end, which are necessary to safely manage, contain, and either reprocess or dispose of spent nuclear fuel. If spent fuel is not reprocessed, the fuel cycle is referred to as an open fuel cycle ; if the spent fuel is reprocessed, it is referred to as a closed fuel cycle.
Mixed oxide fuel, commonly referred to as MOX fuel, is nuclear fuel that contains more than one oxide of fissile material, usually consisting of plutonium blended with natural uranium, reprocessed uranium, or depleted uranium. MOX fuel is an alternative to the low-enriched uranium (LEU) fuel used in the light-water reactors that predominate nuclear power generation.

The Baghdad Nuclear Research Facility adjacent to the Tuwaitha "Yellow Cake Factory" or Tuwaitha Nuclear Research Center contains the remains of nuclear reactors bombed by Iran in 1980, Israel in 1981 and the United States in 1991. It was used as a storage facility for spent reactor fuel and industrial and medical wastes. The radioactive material would not be useful for a fission bomb, but could be used in a dirty bomb. Following the 2003 invasion of Iraq, the facility was heavily looted by hundreds of Iraqis, though it is unclear what was taken.

A breeder reactor is a nuclear reactor that generates more fissile material than it consumes. Breeder reactors achieve this because their neutron economy is high enough to create more fissile fuel than they use, by irradiation of a fertile material, such as uranium-238 or thorium-232, that is loaded into the reactor along with fissile fuel. Breeders were at first found attractive because they made more complete use of uranium fuel than light water reactors, but interest declined after the 1960s as more uranium reserves were found, and new methods of uranium enrichment reduced fuel costs.

Uranium-238 is the most common isotope of uranium found in nature, with a relative abundance of 99%. Unlike uranium-235, it is non-fissile, which means it cannot sustain a chain reaction in a thermal-neutron reactor. However, it is fissionable by fast neutrons, and is fertile, meaning it can be transmuted to fissile plutonium-239. 238U cannot support a chain reaction because inelastic scattering reduces neutron energy below the range where fast fission of one or more next-generation nuclei is probable. Doppler broadening of 238U's neutron absorption resonances, increasing absorption as fuel temperature increases, is also an essential negative feedback mechanism for reactor control.

Low-level waste (LLW) or Low-level radioactive waste (LLRW) is nuclear waste that does not fit into the categorical definitions for intermediate-level waste (ILW), high-level waste (HLW), spent nuclear fuel (SNF), transuranic waste (TRU), or certain byproduct materials known as 11e(2) wastes, such as uranium mill tailings. In essence, it is a definition by exclusion, and LLW is that category of radioactive wastes that do not fit into the other categories. If LLW is mixed with hazardous wastes as classified by RCRA, then it has a special status as mixed low-level waste (MLLW) and must satisfy treatment, storage, and disposal regulations both as LLW and as hazardous waste. While the bulk of LLW is not highly radioactive, the definition of LLW does not include references to its activity, and some LLW may be quite radioactive, as in the case of radioactive sources used in industry and medicine.

Uranium-234 is an isotope of uranium. In natural uranium and in uranium ore, 234U occurs as an indirect decay product of uranium-238, but it makes up only 0.0055% of the raw uranium because its half-life of just 245,500 years is only about 1/18,000 as long as that of 238U. Thus the rate of 234
U to 238
U in a natural sample is equivalent to the rate of their half lives to one another. The primary path of production of 234U via nuclear decay is as follows: uranium-238 nuclei emit an alpha particle to become thorium-234. Next, with a short half-life, 234Th nuclei emit a beta particle to become protactinium-234 (234Pa), or more likely a nuclear isomer denoted 234mPa. Finally, 234Pa or 234mPa nuclei emit another beta particle to become 234U nuclei.
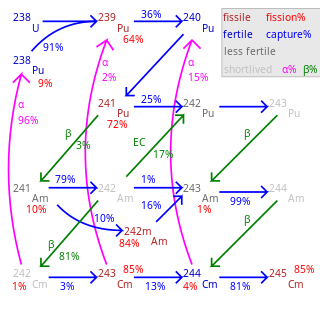
Fertile material is a material that, although not itself fissionable by thermal neutrons, can be converted into a fissile material by neutron absorption and subsequent nuclei conversions.
Uranium (92U) is a naturally occurring radioactive element that has no stable isotope. It has two primordial isotopes, uranium-238 and uranium-235, that have long half-lives and are found in appreciable quantity in the Earth's crust. The decay product uranium-234 is also found. Other isotopes such as uranium-233 have been produced in breeder reactors. In addition to isotopes found in nature or nuclear reactors, many isotopes with far shorter half-lives have been produced, ranging from 214U to 242U. The standard atomic weight of natural uranium is 238.02891(3).

Special nuclear material (SNM) is a term used by the Nuclear Regulatory Commission of the United States to classify fissile materials. The NRC divides special nuclear material into three main categories, according to the risk and potential for its direct use in a clandestine nuclear weapon or for its use in the production of nuclear material for use in a nuclear weapon.

The thorium fuel cycle is a nuclear fuel cycle that uses an isotope of thorium, 232
Th
, as the fertile material. In the reactor, 232
Th
is transmuted into the fissile artificial uranium isotope 233
U
which is the nuclear fuel. Unlike natural uranium, natural thorium contains only trace amounts of fissile material, which are insufficient to initiate a nuclear chain reaction. Additional fissile material or another neutron source is necessary to initiate the fuel cycle. In a thorium-fuelled reactor, 232
Th
absorbs neutrons to produce 233
U
. This parallels the process in uranium breeder reactors whereby fertile 238
U
absorbs neutrons to form fissile 239
Pu
. Depending on the design of the reactor and fuel cycle, the generated 233
U
either fissions in situ or is chemically separated from the used nuclear fuel and formed into new nuclear fuel.

Spent nuclear fuel, occasionally called used nuclear fuel, is nuclear fuel that has been irradiated in a nuclear reactor. It is no longer useful in sustaining a nuclear reaction in an ordinary thermal reactor and depending on its point along the nuclear fuel cycle, it may have considerably different isotopic constituents.
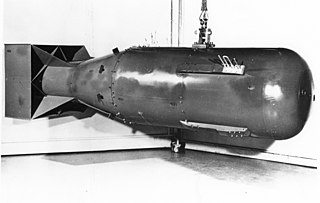
Weapons-grade nuclear material is any fissionable nuclear material that is pure enough to make a nuclear weapon or has properties that make it particularly suitable for nuclear weapons use. Plutonium and uranium in grades normally used in nuclear weapons are the most common examples.
Uranium-236 (236U) is an isotope of uranium that is neither fissile with thermal neutrons, nor very good fertile material, but is generally considered a nuisance and long-lived radioactive waste. It is found in spent nuclear fuel and in the reprocessed uranium made from spent nuclear fuel.

Nuclear transmutation is the conversion of one chemical element or an isotope into another chemical element. Nuclear transmutation occurs in any process where the number of protons or neutrons in the nucleus of an atom is changed.
Depleted uranium hexafluoride (DUHF; also referred to as depleted uranium tails, depleted uranium tailings or DUF6) is a byproduct of the processing of uranium hexafluoride into enriched uranium. It is one of the chemical forms of depleted uranium (up to 73-75%), along with depleted triuranium octoxide (up to 25%) and depleted uranium metal (up to 2%). DUHF is 1.7 times less radioactive than uranium hexafluoride and natural uranium.
References
- ↑ IAEA Safeguards Glossary, sections 4.1, 4.4, 4.5
- ↑ "Nuclear Materials". 2019-01-08.
- ↑ Convention text Archived 2008-02-13 at the Wayback Machine
- ↑ "How We Regulate". 2017-12-15.
- ↑ "Nuclear Materials". Energy.gov. Retrieved 2020-10-17.
- ↑ US EPA, OP (2013-02-22). "Summary of the Nuclear Waste Policy Act". US EPA. Retrieved 2020-10-17.