
Polyurethane refers to a class of polymers composed of organic units joined by carbamate (urethane) links. In contrast to other common polymers such as polyethylene and polystyrene, polyurethane is produced from a wide range of starting materials. This chemical variety produces polyurethanes with different chemical structures leading to many different applications. These include rigid and flexible foams, and coatings, adhesives, electrical potting compounds, and fibers such as spandex and polyurethane laminate (PUL). Foams are the largest application accounting for 67% of all polyurethane produced in 2016.
Ferrocene is an organometallic compound with the formula Fe(C5H5)2. The molecule is a complex consisting of two cyclopentadienyl rings sandwiching a central iron atom. It is an orange solid with a camphor-like odor that sublimes above room temperature, and is soluble in most organic solvents. It is remarkable for its stability: it is unaffected by air, water, strong bases, and can be heated to 400 °C without decomposition. In oxidizing conditions it can reversibly react with strong acids to form the ferrocenium cation Fe(C5H5)+2. Ferrocene and the ferrocenium cation are sometimes abbreviated as Fc and Fc+ respectively.

In organic chemistry, a carbamate is a category of organic compounds with the general formula R2NC(O)OR and structure >N−C(=O)−O−, which are formally derived from carbamic acid. The term includes organic compounds, formally obtained by replacing one or more of the hydrogen atoms by other organic functional groups; as well as salts with the carbamate anion H2NCOO−.
In organic chemistry, a polyol is an organic compound containing multiple hydroxyl groups. The term "polyol" can have slightly different meanings depending on whether it is used in food science or polymer chemistry. Polyols containing two, three and four hydroxyl groups are diols, triols, and tetrols, respectively.
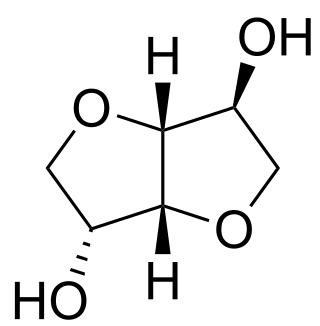
Isosorbide is a bicyclic chemical compound from the group of diols and the oxygen-containing heterocycles, containing two fused furan rings. The starting material for isosorbide is D-sorbitol, which is obtained by catalytic hydrogenation of D-glucose, which is in turn produced by hydrolysis of starch. Isosorbide is discussed as a plant-based platform chemical from which biodegradable derivatives of various functionality can be obtained.

1,1′-Bis(diphenylphosphino)ferrocene, commonly abbreviated dppf, is an organophosphorus compound commonly used as a ligand in homogeneous catalysis. It contains a ferrocene moiety in its backbone, and is related to other bridged diphosphines such as 1,2-bis(diphenylphosphino)ethane (dppe).

Methyltrichlorosilane, also known as trichloromethylsilane, is a monomer and organosilicon compound with the formula CH3SiCl3. It is a colorless liquid with a sharp odor similar to that of hydrochloric acid. As methyltrichlorosilane is a reactive compound, it is mainly used a precursor for forming various cross-linked siloxane polymers.
Biodegradable polymers are a special class of polymer that breaks down after its intended purpose by bacterial decomposition process to result in natural byproducts such as gases (CO2, N2), water, biomass, and inorganic salts. These polymers are found both naturally and synthetically made, and largely consist of ester, amide, and ether functional groups. Their properties and breakdown mechanism are determined by their exact structure. These polymers are often synthesized by condensation reactions, ring opening polymerization, and metal catalysts. There are vast examples and applications of biodegradable polymers.
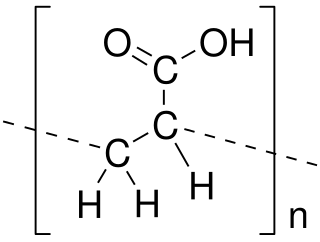
Poly(acrylic acid) (PAA; trade name Carbomer) is a polymer with the formula (CH2-CHCO2H)n. It is a derivative of acrylic acid (CH2=CHCO2H). In addition to the homopolymers, a variety of copolymers and crosslinked polymers, and partially deprotonated derivatives thereof are known and of commercial value. In a water solution at neutral pH, PAA is an anionic polymer, i.e., many of the side chains of PAA lose their protons and acquire a negative charge. Partially or wholly deprotonated PAAs are polyelectrolytes, with the ability to absorb and retain water and swell to many times their original volume. These properties – acid-base and water-attracting – are the bases of many applications.

A silsesquioxane is an organosilicon compound with the chemical formula [RSiO3/2]n. Silsesquioxanes are colorless solids that adopt cage-like or polymeric structures with Si-O-Si linkages and tetrahedral Si vertices. Silsesquioxanes are members of polyoctahedral silsesquioxanes ("POSS"), which have attracted attention as preceramic polymer precursors to ceramic materials and nanocomposites. Diverse substituents (R) can be attached to the Si centers. The molecules are unusual because they feature an inorganic silicate core and an organic exterior. The silica core confers rigidity and thermal stability.

Sodium cyclopentadienide is an organosodium compound with the formula C5H5Na. The compound is often abbreviated as NaCp, where Cp− is the cyclopentadienide anion. Sodium cyclopentadienide is a colorless solid, although samples often are pink owing to traces of oxidized impurities.
Organoiron chemistry is the chemistry of iron compounds containing a carbon-to-iron chemical bond. Organoiron compounds are relevant in organic synthesis as reagents such as iron pentacarbonyl, diiron nonacarbonyl and disodium tetracarbonylferrate. Although iron is generally less active in many catalytic applications, it is less expensive and "greener" than other metals. Organoiron compounds feature a wide range of ligands that support the Fe-C bond; as with other organometals, these supporting ligands prominently include phosphines, carbon monoxide, and cyclopentadienyl, but hard ligands such as amines are employed as well.
In polymer chemistry, the term prepolymer or pre-polymer, refers to a monomer or system of monomers that have been reacted to an intermediate-molecular mass state. This material is capable of further polymerization by reactive groups to a fully cured, high-molecular-mass state. As such, mixtures of reactive polymers with un-reacted monomers may also be referred to as pre-polymers. The term "pre-polymer" and "polymer precursor" may be interchanged.

Polybutylene succinate (PBS) is a thermoplastic polymer resin of the polyester family. PBS is a biodegradable aliphatic polyester with properties that are comparable to polypropylene.

Smart inorganic polymers (SIPs) are hybrid or fully inorganic polymers with tunable (smart) properties such as stimuli responsive physical properties (shape, conductivity, rheology, bioactivity, self-repair, sensing etc.). While organic polymers are often petrol-based, the backbones of SIPs are made from elements other than carbon which can lessen the burden on scarce non-renewable resources and provide more sustainable alternatives. Common backbones utilized in SIPs include polysiloxanes, polyphosphates, and polyphosphazenes, to name a few.
Polyorthoesters are polymers with the general structure –[–R–O–C(R1, OR2)–O–R3–]n– whereas the residue R2 can also be part of a heterocyclic ring with the residue R. Polyorthoesters are formed by transesterification of orthoesters with diols or by polyaddition between a diol and a diketene acetal, such as 3,9-diethylidene-2,4,8,10-tetraoxaspiro[5.5]undecane.
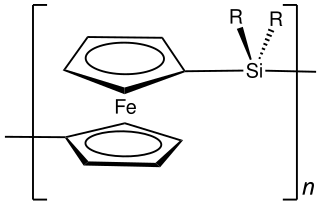
Polyferrocenes are polymers containing ferrocene units. Ferrocene offers many advantages over pure hydrocarbons when used as a building block of macromolecular chemistry. The variety of possible substitutions at the ferrocene parent body results in a multitude of accessible polymers with interesting electronic and photonic properties. Many polyferrocenes are relatively easily accessible. Poly(1,1'-ferrocene-silane) can be prepared by ring-opening polymerization and has a variety of interesting properties, such as a high refractive index or semiconductor properties. Ring-opening polymerization usually leads to polymers containing ferrocene in the backbone. Besides the latter motif, ferrocene can be attached to the backbone as pendant unit as well.
1,1'-Dilithioferrocene is the organoiron compound with the formula Fe(C5H4Li)2. It is exclusively generated and isolated as a solvate, using either ether or tertiary amine ligands bound to the lithium centers. Regardless of the solvate, dilithioferrocene is used commonly to prepare derivatives of ferrocene.

1,1'-Ferrocenedicarboxylic acid is the organoiron compound with the formula Fe(C5H4CO2H)2. It is the simplest dicarboxylic acid derivative of ferrocene. It is a yellow solid that is soluble in aqueous base. The 1,1' part of its name refers to the location of the carboxylic acid groups on separate rings.
1,1'-Diaminoferrocene is the organoiron compound with the formula Fe(C5H4NH2)2. It is the simplest diamine derivative of ferrocene. It is a yellow, air-sensitive solid that is soluble in aqueous acid. The 1,1' part of its name refers to the location of the amine groups on separate rings. Compared to the parent ferrocene, the diamine is about 600 mV more reducing.












