
Naphthalene is an organic compound with formula C
10H
8. It is the simplest polycyclic aromatic hydrocarbon, and is a white crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 ppm by mass. As an aromatic hydrocarbon, naphthalene's structure consists of a fused pair of benzene rings. It is the main ingredient of traditional mothballs.

1,4-Dichlorobenzene (1,4-DCB, p-DCB, or para-dichlorobenzene, sometimes abbreviated as PDCB or para) is an aryl chloride and isomer of dichlorobenzene with the formula C6H4Cl2. This colorless solid has a strong odor. The molecule consists of a benzene ring with two chlorine atoms (replacing hydrogen atoms) on opposing sites of the ring.

Phthalic anhydride is the organic compound with the formula C6H4(CO)2O. It is the anhydride of phthalic acid. Phthalic anhydride is a principal commercial form of phthalic acid. It was the first anhydride of a dicarboxylic acid to be used commercially. This white solid is an important industrial chemical, especially for the large-scale production of plasticizers for plastics. In 2000, the worldwide production volume was estimated to be about 3 million tonnes per year.

Acenaphthene is a polycyclic aromatic hydrocarbon (PAH) consisting of naphthalene with an ethylene bridge connecting positions 1 and 8. It is a colourless solid. Coal tar consists of about 0.3% of this compound.
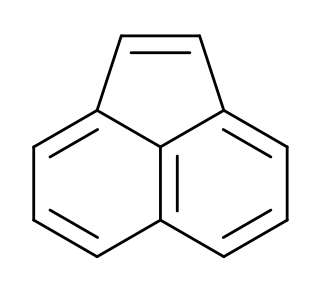
Acenaphthylene, a polycyclic aromatic hydrocarbon is an ortho- and peri-fused tricyclic hydrocarbon. The molecule resembles naphthalene with positions 1 and 8 connected by a -CH=CH- unit. It is a yellow solid. Unlike many polycyclic aromatic hydrocarbons, it has no fluorescence.

Decalin, a bicyclic organic compound, is an industrial solvent. A colorless liquid with an aromatic odor, it is used as a solvent for many resins or fuel additives.
Cycloocta-1,5-diene is a cyclic hydrocarbon with the chemical formula C8H12, specifically [−(CH2)2−CH=CH−]2.
Pyrazolone is 5-membered heterocycle containing two adjacent nitrogen atoms. It can be viewed as a derivative of pyrazole possessing an additional carbonyl (C=O) group. Compounds containing this functional group are useful commercially in analgesics and dyes.
The Bucherer reaction in organic chemistry is the reversible conversion of a naphthol to a naphthylamine in the presence of ammonia and sodium bisulfite. The reaction is widely used in the synthesis of dye precursors aminonaphthalenesulfonic acids.

Armstrong's acid (naphthalene-1,5-disulfonic acid) is a fluorescent organic compound with the formula C10H6(SO3H)2. It is one of several isomers of naphthalenedisulfonic acid. It a colorless solid, typically obtained as the tetrahydrate. Like other sulfonic acids, it is a strong acid. It is named for British chemist Henry Edward Armstrong.

2-Naphthol, or β-naphthol, is a fluorescent colorless (or occasionally yellow) crystalline solid with the formula C10H7OH. It is an isomer of 1-naphthol, differing by the location of the hydroxyl group on the naphthalene ring. The naphthols are naphthalene homologues of phenol, but more reactive. Both isomers are soluble in simple alcohols, ethers, and chloroform. 2-Naphthol is a widely used intermediate for the production of dyes and other compounds.
1-Naphthol, or α-naphthol, is a organic compound with the formula C10H7OH. It is a fluorescent white solid. 1-Naphthol differs from its isomer 2-naphthol by the location of the hydroxyl group on the naphthalene ring. The naphthols are naphthalene homologues of phenol. Both isomers are soluble in simple organic solvents. They are precursors to a variety of useful compounds.
4-Nitrotoluene or para-nitrotoluene is an organic compound with the formula CH3C6H4NO2. It is a pale yellow solid. It is one of three isomers of nitrotoluene.

1,4-Naphthoquinone or para-naphthoquinone is a quinone derived from naphthalene. It forms volatile yellow triclinic crystals and has a sharp odor similar to benzoquinone. It is almost insoluble in cold water, slightly soluble in petroleum ether, and more soluble in polar organic solvents. In alkaline solutions it produces a reddish-brown color. Vitamin K is a derivative of 1,4-naphthoquinone. It is a planar molecule with one aromatic ring fused to a quinone subunit. It is an isomer of 1,2-naphthoquinone.
Naphthalenetetracarboxylic dianhydride (NTDA) is an organic compound related to naphthalene. The compound is a beige solid. NTDA is most commonly used as a precursor to naphthalenediimides (NDIs), a family of compounds with many uses.

2,6-Dimethylnaphthalene (2,6-DMN) is a polycyclic aromatic hydrocarbon. It is one of the ten dimethylnaphthalene isomers, which are derived from naphthalene by the addition of two methyl groups.

Naphthalene-1-sulfonic acid is an organic compound with the formula C10H7SO3H. A colorless, water-soluble solid, it is often available as the dihydrate C10H7SO3H.2H2O. It is one of two monosulfonic acids of naphthalene, the other being the more stable naphthalene-2-sulfonic acid. The compound is mainly used in the production of dyes.

Naphthalene-2-sulfonic acid is an organic compound with the formula C10H7SO3H. A colorless, water-soluble solid, it is often available as the mono- and trihydrates C10H7SO3H.2H2O. It is one of two monosulfonic acids of naphthalene, the other being naphthalene-1-sulfonic acid. The compound is mainly used in the production of dyes via nitration en route to aminonaphthalenesulfonic acids. The compound is prepared by sulfonation of naphthalene with sulfuric acid, however under equilibrating conditions that allow the 1-sulfonic acid isomer to convert to the more stable 2-sulfonic acid.

1,5-Diaminonaphthalene is an organic compound with the formula C10H6(NH2)2. It is one of several diaminonaphthalenes. It is a colorless solid that darkens in air due to oxidation.
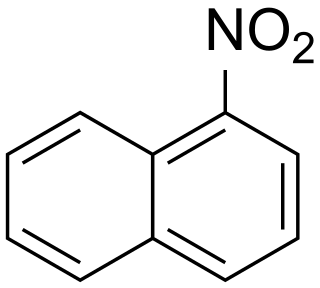
1-Nitronaphthalene is an organic compound with the formula C10H7NO2. It is one of two isomers of nitronaphthalene. A pale yellow, sublimable solid, 1-nitronaphthalene is the main product of the direct nitration of naphthalene. It is an intermediate in the production of naphthylamine, a precursor to dyes. The conversion to the amine is effected by hydrogenation.














