
Annulenes are monocyclic hydrocarbons that contain the maximum number of non-cumulated double bonds ('mancude'). They have the general formula CnHn or CnHn+1. The IUPAC naming conventions are that annulenes with 7 or more carbon atoms are named as [n]annulene, where n is the number of carbon atoms in their ring, though sometimes the smaller annulenes are referred to using the same notation, and benzene is sometimes referred to simply as annulene.
In organic chemistry, Hückel's rule estimates whether a planar ring molecule will have aromatic properties. The quantum mechanical basis for its formulation was first worked out by physical chemist Erich Hückel in 1931. The succinct expression as the 4n + 2 rule has been attributed to W. v. E. Doering (1951), although several authors were using this form at around the same time.
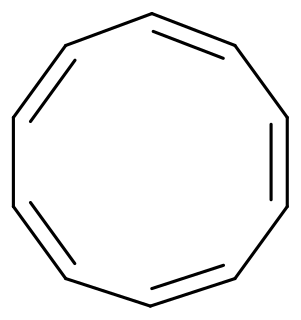
Cyclodecapentaene or [10]annulene is an annulene with molecular formula C10H10. This organic compound is a conjugated 10 pi electron cyclic system and according to Huckel's rule it should display aromaticity. It is not aromatic, however, because various types of ring strain destabilize an all-planar geometry. The all-cis isomer (1), a fully convex decagon, would have bond angles of 144°, which creates large amounts of angle strain relative to the ideal 120° for sp2 atomic hybridization. Instead, the all-cis isomer can adopt a planar boat-like conformation (2) to relieve the angle strain. This is still unstable because of the relative higher strain in boat shaped compared to the next planar trans, cis, trans, cis, cis isomer (3). Yet even this isomer is also unstable, suffering from steric repulsion between the two internal hydrogen atoms. The nonplanar trans, cis, cis, cis, cis isomer (4) is the most stable of all the possible isomers.
Dichlorocarbene is the reactive intermediate with chemical formula CCl2. Although this chemical species has not been isolated, it is a common intermediate in organic chemistry, being generated from chloroform. This bent diamagnetic molecule rapidly inserts into other bonds.

Cyclooctadecanonaene or [18]annulene is an organic compound with chemical formula C
18H
18. It belongs to the class of highly conjugated compounds known as annulenes and is aromatic. The usual isomer that [18]annulene refers to is the most stable one, containing six interior hydrogens and twelve exterior ones, with the nine formal double bonds in the cis,trans,trans,cis,trans,trans,cis,trans,trans configuration. It is reported to be a red-brown crystalline solid.

The Bingel reaction in fullerene chemistry is a fullerene cyclopropanation reaction to a methanofullerene first discovered by C. Bingel in 1993 with the bromo derivative of diethyl malonate in the presence of a base such as sodium hydride or DBU. The preferred double bonds for this reaction on the fullerene surface are the shorter bonds at the junctions of two hexagons and the driving force is relief of steric strain.

Annulynes or dehydroannulenes are conjugated monocyclic hydrocarbons with alternating single and double bonds in addition to at least one triple bond.
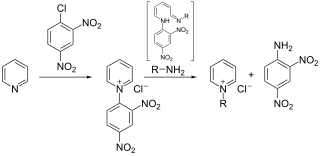
The Zincke reaction is an organic reaction in which a pyridine is transformed into a pyridinium salt by reaction with 2,4-dinitro-chlorobenzene and a primary amine, named after Theodor Zincke.

Cyclododecahexaene or [12]annulene is a member of the series of annulenes with some interest in organic chemistry with regard to the study of aromaticity. Cyclododecahexaene is non-aromatic due to the lack of planarity of the structure. On the other hand the dianion with 14 electrons is a Hückel aromat and more stable.
Edgar Heilbronner was a Swiss German chemist. In 1964 he published the concept of Möbius cyclic annulenes, but the first Möbius aromatic was not synthesized until 2003.

2-Diphenylmethylpyrrolidine (Desoxy-D2PM), also known as 2-benzhydrylpyrrolidine, is a stimulant psychoactive drug. It is the 4-dehydroxylated structural analog of diphenylprolinol (D2PM), and is also similar in structure to desoxypipradrol (2-DPMP), both of which act as norepinephrine-dopamine reuptake inhibitors (NDRIs). Like D2PM and 2-DPMP, Desoxy-D2PM is sold as a designer drug and has been used in the manufacture of legal highs. It has been marketed under the names A3A New Generation, A3A Methano, and Green Powder, and has been reported to cause hallucinations, violent behavior, dilated pupils, tachycardia, and high blood pressure. Literature data suggest that it can produce the same psychotropic effects as other stimulants, but with a longer duration of action.
The molecular formula C11H10 may refer to:

Benzomorphan is a chemical compound that is the base for a series of drugs which variably act on the opioid kappa and sigma receptors, including the following compounds:

Zethrene (dibenzo[de,mn]naphthacene) is a polycyclic aromatic hydrocarbon consisting of two phenalene units fused together. According to Clar's rule, the two exterior naphthalene units are truly aromatic and the two central double bonds are not aromatic at all. For this reason the compound is of some interest to academic research. Zethrene has a deep-red color and it is light sensitive - complete decomposition under a sunlight lamp occurs within 12 hours. The melting point is 262 °C.
Cyclotetradecaheptaene, often referred to as [14]annulene, is a hydrocarbon with molecular formula C14H14, which played an important role in the development of criteria (Hückel's rule) for aromaticity, a stabilizing property of central importance in physical organic chemistry. It forms dark-red needle-like crystals.
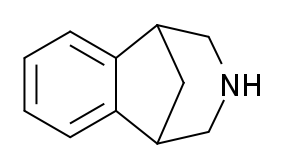
2,3,4,5-Tetrahydro-1,5-methano-1H-3-benzazepine is a drug originally researched as a potential opioid analgesic, but was found to be inactive in this assay, and relatively toxic to mice. Subsequently it was found to possess activity as an agonist at nicotinic acetylcholine receptors during the course of work that ultimately led to the discovery of the anti-smoking drug varenicline.

8-Carboxamidocyclazocine (8-CAC) is an opioid analgesic drug related to cyclazocine, discovered by medicinal chemist Mark P. Wentland and co-workers in Cogswell Laboratory at Rensselaer Polytechnic Institute. Similarly to cyclazocine, 8-CAC acts as an agonist at both the μ and κ opioid receptors, but has a much longer duration of action than cyclazocine, and does not have μ antagonist activity. Unexpectedly it was discovered that the phenolic hydroxyl group of cyclazocine could be replaced by a carboxamido group with only slight loss of potency at opioid receptors, and this discovery has subsequently been used to develop many novel opioid derivatives where the phenolic hydroxy group has been replaced by either carboxamide or a variety of larger groups. Due to their strong κ-opioid agonist activity, these drugs are not suited for use as analgesics in humans, but have instead been researched as potential drugs for the treatment of cocaine addiction.
In organic chemistry, Baird's rule estimates whether the lowest triplet state of planar, cyclic structures will have aromatic properties or not. The quantum mechanical basis for its formulation was first worked out by physical chemist N. Colin Baird at the University of Western Ontario in 1972.

1,6-Methano[10]annulene (also known as 1,6-methanonaphthalene or homonaphthalene) is an aromatic hydrocarbon with chemical formula C11H10. It was the first stable aromatic compound based on the cyclodecapentaene system to be discovered.
Methano(10)annulene may refer to:













