
The Misuse of Drugs Act 1971 is an act of the Parliament of the United Kingdom. It represents action in line with treaty commitments under the Single Convention on Narcotic Drugs, the Convention on Psychotropic Substances, and the United Nations Convention Against Illicit Traffic in Narcotic Drugs and Psychotropic Substances.
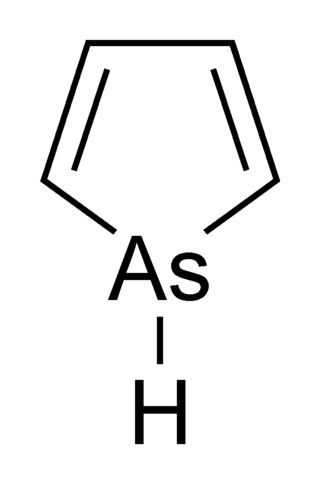
Arsole, also called arsenole or arsacyclopentadiene, is an organoarsenic compound with the formula C4H5As. It is classified as a metallole and is isoelectronic to and related to pyrrole except that an arsenic atom is substituted for the nitrogen atom. Whereas the pyrrole molecule is planar, the arsole molecule is not, and the hydrogen atom bonded to arsenic extends out of the molecular plane. Arsole is only moderately aromatic, with about 40% the aromaticity of pyrrole. Arsole itself has not been reported in pure form, but several substituted analogs called arsoles exist. Arsoles and more complex arsole derivatives have similar structure and chemical properties to those of phosphole derivatives. When arsole is fused to a benzene ring, this molecule is called arsindole, or benzarsole.
Phosphole is the organic compound with the chemical formula C
4H
4PH; it is the phosphorus analog of pyrrole. The term phosphole also refers to substituted derivatives of the parent heterocycle. These compounds are of theoretical interest but also serve as ligands for transition metals and as precursors to more complex organophosphorus compounds.
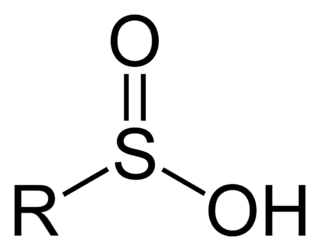
Sulfinic acids are oxoacids of sulfur with the structure RSO(OH). In these organosulfur compounds, sulfur is pyramidal.
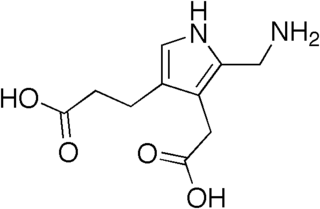
Porphobilinogen (PBG) is an organic compound that occurs in living organisms as an intermediate in the biosynthesis of porphyrins, which include critical substances like hemoglobin and chlorophyll.

Uroporphyrinogen III is a tetrapyrrole, the first macrocyclic intermediate in the biosynthesis of heme, chlorophyll, vitamin B12, and siroheme. It is a colorless compound, like other porphyrinogens.
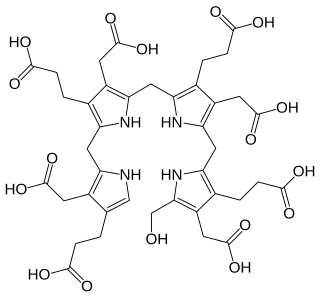
Hydroxymethylbilane, also known as preuroporphyrinogen, is an organic compound that occurs in living organisms during the synthesis of porphyrins, a group of critical substances that include haemoglobin, myoglobin, and chlorophyll. The name is often abbreviated as HMB.
In organic chemistry, the Paal–Knorr synthesis is a reaction used to synthesize substituted furans, pyrroles, or thiophenes from 1,4-diketones. It is a synthetically valuable method for obtaining substituted furans and pyrroles, which are common structural components of many natural products. It was initially reported independently by German chemists Carl Paal and Ludwig Knorr in 1884 as a method for the preparation of furans, and has been adapted for pyrroles and thiophenes. Although the Paal–Knorr synthesis has seen widespread use, the mechanism wasn't fully understood until it was elucidated by V. Amarnath et al. in the 1990s.
In organic chemistry, bilane is a compound with the formula C19H20N4 or [(C4H4N)−CH2−(C4H3N)−]2CH2. It is a tetrapyrrole, a class of compounds with four independent pyrrole rings. Specifically, the molecule can be described as four pyrrole molecules C4H5N connected in an open chain by three methylene bridges −CH2− at carbons adjacent to the nitrogens, replacing the respective hydrogens.
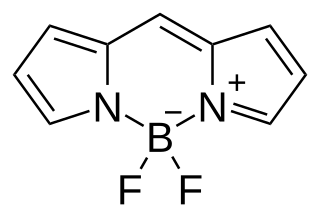
BODIPY is the technical common name of a chemical compound with formula C
9H
7BN
2F
2, whose molecule consists of a boron difluoride group BF
2 joined to a dipyrromethene group C
9H
7N
2; specifically, the compound 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene in the IUPAC nomenclature. The common name is an abbreviation for "boron-dipyrromethene". It is a red crystalline solid, stable at ambient temperature, soluble in methanol.
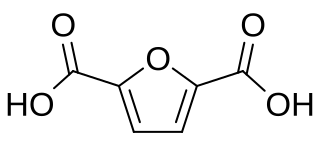
2,5-Furandicarboxylic acid (FDCA) is an organic chemical compound consisting of two carboxylic acid groups attached to a central furan ring. It was first reported as dehydromucic acid by Rudolph Fittig and Heinzelmann in 1876, who produced it via the action of concentrated hydrobromic acid upon mucic acid. It can be produced from certain carbohydrates and as such is a renewable resource, it was identified by the US Department of Energy as one of 12 priority chemicals for establishing the “green” chemistry industry of the future. Furan-2,5-dicarboxylic acid (FDCA) has been suggested as an important renewable building block because it can substitute for terephthalic acid (PTA) in the production of polyesters and other current polymers containing an aromatic moiety.

The Rothemund reaction is a condensation/oxidation process that converts four pyrroles and four aldehydes into a porphyrin. It is based on work by Paul Rothemund, who first reported it in 1936. The method underpins more modern synthesis such as those described by Adler and Longo and by Lindsey. The Rothemund reactions is common in university teaching labs.

Stibole is a theoretical heterocyclic organic compound, a five-membered ring with the formula C4H4SbH. It is classified as a metallole. It can be viewed as a structural analog of pyrrole, with antimony replacing the nitrogen atom of pyrrole. Substituted derivatives, which have been synthesized, are called stiboles.

Bismole is a theoretical heterocyclic organic compound, a five-membered ring with the formula C4H4BiH. It is classified as a metallole. It can be viewed as a structural analog of pyrrole, with bismuth replacing the nitrogen atom of pyrrole. The unsubstituted compound has not been isolated due to the high energy of the Bi-H bond. Substituted derivatives, which have been synthesized, are called bismoles.
The molecular formula C6H9NO2 (molar mass: 127.14 g/mol, exact mass: 127.0633 u) may refer to:

HU-243 (AM-4056) is a synthetic cannabinoid drug that is a single enantiomer of the hydrogenated derivative of the commonly used reference agonist HU-210. It is a methylene homologue of canbisol. It is a potent agonist at both the CB1 and CB2 receptors, with a binding affinity of 0.041 nM at the CB1 receptor, making it marginally more potent than HU-210, which had an affinity of 0.061 nM in the same assay.

2,5-Bis(hydroxymethyl)furan (BHMF) is a heterocyclic organic compound, and is a derivative of a broader class of compounds known as furans. It is produced from cellulose and has received attention as a biofeedstock. It is a white solid, although commercial samples can appear yellowish or tan.

2,2'-Dipyrromethene, often called just dipyrromethene or dipyrrin, is a chemical compound with formula C
9H
8N
2 whose skeleton can be described as two pyrrole rings C
5N connected by a methyne bridge =CH– through their nitrogen-adjacent (position-2) carbons; the remaining bonds being satisfied by hydrogen atoms. It is an unstable compound that is readily attacked by nucleophilic compounds above −40 °C.
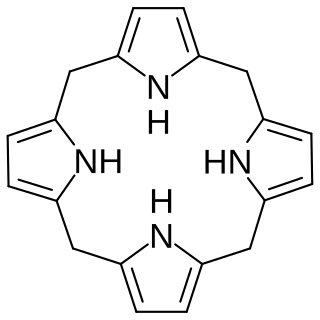
Hexahydroporphine is an organic chemical compound with formula C20H20N4. The molecule consists of four pyrrole rings connected by methylene bridges −CH2− into a larger (non-aromatic) macrocycle ring, which makes it one of the simplest tetrapyrroles, and the simplest "true" one. As indicated by the name, it may be viewed as derived from porphine by the addition of six hydrogen atoms: four on the methine bridges, and two on the nitrogen atoms.

Phosphorus-centered porphyrins are conjugated polycyclic ring systems consisting of either four pyrroles with inward-facing nitrogens and a phosphorus atom at their core or porphyrins with one of the four pyrroles substituted for a phosphole. Unmodified porphyrins are composed of pyrroles and linked by unsaturated hydrocarbon bridges often acting as multidentate ligands centered around a transition metal like Cu II, Zn II, Co II, Fe III. Being highly conjugated molecules with many accessible energy levels, porphyrins are used in biological systems to perform light-energy conversion and modified synthetically to perform similar functions as a photoswitch or catalytic electron carriers. Phosphorus III and V ions are much smaller than the typical metal centers and bestow distinct photochemical properties unto the porphyrin. Similar compounds with other pnictogen cores or different polycyclic rings coordinated to phosphorus result in other changes to the porphyrin’s chemistry.
















