
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms. Study of structure determines their structural formula. Study of properties includes physical and chemical properties, and evaluation of chemical reactivity to understand their behavior. The study of organic reactions includes the chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical study.

Indigo dye is an organic compound with a distinctive blue color. Indigo is a natural dye extracted from the leaves of some plants of the Indigofera genus, in particular Indigofera tinctoria. Dye-bearing Indigofera plants were commonly grown and used throughout the world, particularly in Asia, with the production of indigo dyestuff economically important due to the historical rarity of other blue dyestuffs.

Acridine is an organic compound and a nitrogen heterocycle with the formula C13H9N. Acridines are substituted derivatives of the parent ring. It is a planar molecule that is structurally related to anthracene with one of the central CH groups replaced by nitrogen. Like the related molecules pyridine and quinoline, acridine is mildly basic. It is an almost colorless solid, which crystallizes in needles. There are few commercial applications of acridines; at one time acridine dyes were popular, but they are now relegated to niche applications, such as with acridine orange. The name is a reference to the acrid odour and acrid skin-irritating effect of the compound.

Tyrian purple, also known as royal purple, imperial purple, or imperial dye, is a reddish-purple natural dye. The name Tyrian refers to Tyre, Lebanon, once Phoenicia. It is secreted by several species of predatory sea snails in the family Muricidae, rock snails originally known by the name Murex. In ancient times, extracting this dye involved tens of thousands of snails and substantial labour, and as a result, the dye was highly valued.The colored compound is 6,6'-dibromoindigo.

Mauveine, also known as aniline purple and Perkin's mauve, was one of the first synthetic dyes. It was discovered serendipitously by William Henry Perkin in 1856 while he was attempting to synthesise the phytochemical quinine for the treatment of malaria. It is also among the first chemical dyes to have been mass-produced.

Hexaplex trunculus is a medium-sized sea snail, a marine gastropod mollusk in the family Muricidae, the murex shells or rock snails. It is included in the subgenus Trunculariopsis.
Halocarbon compounds are chemical compounds in which one or more carbon atoms are linked by covalent bonds with one or more halogen atoms resulting in the formation of organofluorine compounds, organochlorine compounds, organobromine compounds, and organoiodine compounds. Chlorine halocarbons are the most common and are called organochlorides.

Tekhelet is a highly valued dye described as either "sky blue", or "light blue", that held great significance in ancient Mediterranean civilizations. In the Hebrew Bible and Jewish tradition, tekhelet was used to colour the clothing of the High Priest of Israel, the tapestries in the Tabernacle, and the tzitzit (fringes) attached to the corners of four-cornered garments, including the tallit.

A natural product is a natural compound or substance produced by a living organism—that is, found in nature. In the broadest sense, natural products include any substance produced by life. Natural products can also be prepared by chemical synthesis and have played a central role in the development of the field of organic chemistry by providing challenging synthetic targets. The term natural product has also been extended for commercial purposes to refer to cosmetics, dietary supplements, and foods produced from natural sources without added artificial ingredients.

In stereochemistry, a chiral auxiliary is a stereogenic group or unit that is temporarily incorporated into an organic compound in order to control the stereochemical outcome of the synthesis. The chirality present in the auxiliary can bias the stereoselectivity of one or more subsequent reactions. The auxiliary can then be typically recovered for future use.

Pentacene is a polycyclic aromatic hydrocarbon consisting of five linearly-fused benzene rings. This highly conjugated compound is an organic semiconductor. The compound generates excitons upon absorption of ultra-violet (UV) or visible light; this makes it very sensitive to oxidation. For this reason, this compound, which is a purple powder, slowly degrades upon exposure to air and light.

Paul Friedländer was a German chemist best known for his research on derivates of indigo and isolation of Tyrian purple from Murex brandaris.

Fullerene chemistry is a field of organic chemistry devoted to the chemical properties of fullerenes. Research in this field is driven by the need to functionalize fullerenes and tune their properties. For example, fullerene is notoriously insoluble and adding a suitable group can enhance solubility. By adding a polymerizable group, a fullerene polymer can be obtained. Functionalized fullerenes are divided into two classes: exohedral fullerenes with substituents outside the cage and endohedral fullerenes with trapped molecules inside the cage.

Bolinus brandaris, and commonly known as the purple dye murex or the spiny dye-murex, is a species of medium-sized predatory sea snail, an edible marine gastropod mollusk in the family Muricidae, the murex snails or the rock snails.
Organobromine chemistry is the study of the synthesis and properties of organobromine compounds, also called organobromides, which are organic compounds that contain carbon bonded to bromine. The most pervasive is the naturally produced bromomethane.
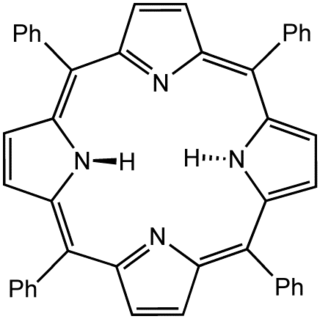
Tetraphenylporphyrin, abbreviated TPP or H2TPP, is a synthetic heterocyclic compound that resembles naturally occurring porphyrins. Porphyrins are dyes and cofactors found in hemoglobin and cytochromes and are related to chlorophyll and vitamin B12. The study of naturally occurring porphyrins is complicated by their low symmetry and the presence of polar substituents. Tetraphenylporphyrin is hydrophobic, symmetrically substituted, and easily synthesized. The compound is a dark purple solid that dissolves in nonpolar organic solvents such as chloroform and benzene.

Indole is an organic compound with the formula C6H4CCNH3. Indole is classified as an aromatic heterocycle. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered pyrrole ring. Indoles are derivatives of indole where one or more of the hydrogen atoms have been replaced by substituent groups. Indoles are widely distributed in nature, most notably as amino acid tryptophan and neurotransmitter serotonin.
1,4-Dibromobenzene (p-dibromobenzene) is an aryl bromide and isomer of dibromobenzene that is solid at room temperature. It has a strong smell similar to that of the lighter chlorine analogue. It can be used as a precursor to the dye 6,6-dibromoindigo.

Dyeing is the craft of imparting colors to textiles in loose fiber, yarn, cloth or garment form by treatment with a dye. Archaeologists have found evidence of textile dyeing with natural dyes dating back to the Neolithic period. In China, dyeing with plants, barks and insects has been traced back more than 5,000 years. Natural insect dyes such as Tyrian purple and kermes and plant-based dyes such as woad, indigo and madder were important elements of the economies of Asia and Europe until the discovery of man-made synthetic dyes in the mid-19th century. Synthetic dyes quickly superseded natural dyes for the large-scale commercial textile production enabled by the Industrial Revolution, but natural dyes remained in use by traditional cultures around the world.

Bromide peroxidase (EC 1.11.1.18, bromoperoxidase, haloperoxidase (ambiguous), eosinophil peroxidase) is a family of enzymes with systematic name bromide:hydrogen-peroxide oxidoreductase. These enzymes catalyse the following chemical reaction:


















