Related Research Articles
Heat shock proteins (HSP) are a family of proteins that are produced by cells in response to exposure to stressful conditions. They were first described in relation to heat shock, but are now known to also be expressed during other stresses including exposure to cold, UV light and during wound healing or tissue remodeling. Many members of this group perform chaperone functions by stabilizing new proteins to ensure correct folding or by helping to refold proteins that were damaged by the cell stress. This increase in expression is transcriptionally regulated. The dramatic upregulation of the heat shock proteins is a key part of the heat shock response and is induced primarily by heat shock factor (HSF). HSPs are found in virtually all living organisms, from bacteria to humans.
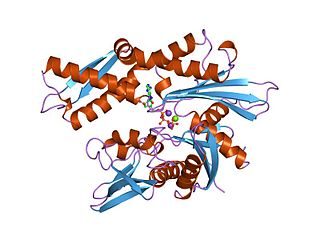
The 70 kilodalton heat shock proteins are a family of conserved ubiquitously expressed heat shock proteins. Proteins with similar structure exist in virtually all living organisms. The Hsp70s are an important part of the cell's machinery for protein folding, and help to protect cells from stress.

Hsp90 is a chaperone protein that assists other proteins to fold properly, stabilizes proteins against heat stress, and aids in protein degradation. It also stabilizes a number of proteins required for tumor growth, which is why Hsp90 inhibitors are investigated as anti-cancer drugs.

Hop, occasionally written HOP, is an abbreviation for Hsp70-Hsp90 Organizing Protein. It functions as a co-chaperone which reversibly links together the protein chaperones Hsp70 and Hsp90.
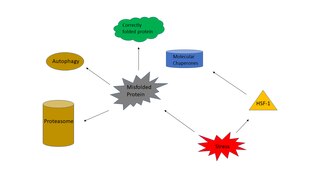
The heat shock response (HSR) is a cellular response that increases the number of molecular chaperones to combat the negative effects on proteins caused by stressors such as increased temperatures, oxidative stress, and heavy metals. In a normal cell, protein homeostasis (proteostasis) must be maintained because proteins are the main functional units of the cell. Proteins take on a defined configuration in order to gain functionality. If these structures are altered, critical processes could be affected, leading to cell damage or death. With the importance of proteins established, the heat shock response can be employed under stress to induce heat shock proteins (HSP), also known as molecular chaperones, that help prevent or reverse protein misfolding and provide an environment for proper folding.

Heat shock 70 kDa protein 8 also known as heat shock cognate 71 kDa protein or Hsc70 or Hsp73 is a heat shock protein that in humans is encoded by the HSPA8 gene on chromosome 11. As a member of the heat shock protein 70 family and a chaperone protein, it facilitates the proper folding of newly translated and misfolded proteins, as well as stabilize or degrade mutant proteins. Its functions contribute to biological processes including signal transduction, apoptosis, autophagy, protein homeostasis, and cell growth and differentiation. It has been associated with an extensive number of cancers, neurodegenerative diseases, cell senescence, and aging.
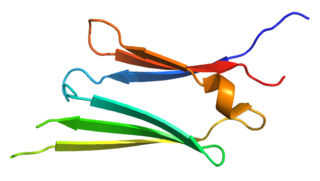
Heat shock protein 27 (Hsp27) also known as heat shock protein beta-1 (HSPB1) is a protein that in humans is encoded by the HSPB1 gene.
Co-chaperones are proteins that assist chaperones in protein folding and other functions. Co-chaperones are the non-client binding molecules that assist in protein folding mediated by Hsp70 and Hsp90. They are particularly essential in stimulation of the ATPase activity of these chaperone proteins. There are a great number of different co-chaperones however based on their domain structure most of them fall into two groups: J-domain proteins and tetratricopeptide repeats (TPR).
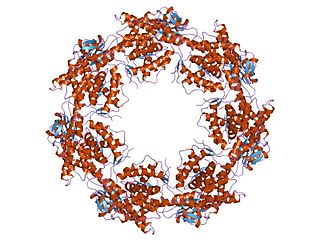
Heat shock proteins are generally responsible for preventing damage to proteins in response to high levels of heat. Heat shock proteins are classified into six major families based on their molecular mass: small HSPs, HSP40, HSP60, HSP70, HSP90, and HSP110

Heat shock 70 kDa protein 1, also termed Hsp72, is a protein that in humans is encoded by the HSPA1A gene. As a member of the heat shock protein 70 family and a chaperone protein, it facilitates the proper folding of newly translated and misfolded proteins, as well as stabilize or degrade mutant proteins. In addition, Hsp72 also facilitates DNA repair. Its functions contribute to biological processes including signal transduction, apoptosis, protein homeostasis, and cell growth and differentiation. It has been associated with an extensive number of cancers, neurodegenerative diseases, cell senescence and aging, and inflammatory diseases such as Diabetes mellitus type 2 and rheumatoid arthritis.

Heat shock protein HSP 90-alpha is a protein that in humans is encoded by the HSP90AA1 gene.

Heat shock factor 1 (HSF1) is a protein that in humans is encoded by the HSF1 gene. HSF1 is highly conserved in eukaryotes and is the primary mediator of transcriptional responses to proteotoxic stress with important roles in non-stress regulation such as development and metabolism.

MAP kinase-activated protein kinase 2 is an enzyme that in humans is encoded by the MAPKAPK2 gene.

Heat shock protein 90kDa beta member 1 (HSP90B1), known also as endoplasmin, gp96, grp94, or ERp99, is a chaperone protein that in humans is encoded by the HSP90B1 gene.

Heat shock protein HSP 90-beta also called HSP90beta is a protein that in humans is encoded by the HSP90AB1 gene.

Prostaglandin E synthase 3 (cytosolic) is an enzyme that in humans is encoded by the PTGES3 gene.

Binding immunoglobulin protein (BiP) also known as (GRP-78) or heat shock 70 kDa protein 5 (HSPA5) or (Byun1) is a protein that in humans is encoded by the HSPA5 gene.

DnaJ homolog subfamily B member 1 is a protein that in humans is encoded by the DNAJB1 gene.
Activator of 90 kDa heat shock protein ATPase homolog 1 is an enzyme that in humans is encoded by the AHSA1 gene.

In molecular biology, chaperone DnaJ, also known as Hsp40, is a molecular chaperone protein. It is expressed in a wide variety of organisms from bacteria to humans.
References
- 1 2 Panaretou B, Siligardi G, Meyer P, Maloney A, Sullivan JK, Singh S, Millson SH, Clarke PA, Naaby-Hansen S, Stein R, Cramer R, Mollapour M, Workman P, Piper PW, Pearl LH, Prodromou C (December 2002). "Activation of the ATPase activity of hsp90 by the stress-regulated cochaperone aha1" (PDF). Mol. Cell. 10 (6): 1307–18. doi:10.1016/S1097-2765(02)00785-2. PMID 12504007.
| This protein-related article is a stub. You can help Wikipedia by expanding it. |