Related Research Articles

The citric acid cycle—also known as the Krebs cycle, Szent-Györgyi-Krebs cycle or the TCA cycle (tricarboxylic acid cycle)—is a series of biochemical reactions to release the energy stored in nutrients through the oxidation of acetyl-CoA derived from carbohydrates, fats, and proteins. The chemical energy released is available under the form of ATP. The Krebs cycle is used by organisms that respire (as opposed to organisms that ferment) to generate energy, either by anaerobic respiration or aerobic respiration. In addition, the cycle provides precursors of certain amino acids, as well as the reducing agent NADH, that are used in numerous other reactions. Its central importance to many biochemical pathways suggests that it was one of the earliest components of metabolism. Even though it is branded as a 'cycle', it is not necessary for metabolites to follow only one specific route; at least three alternative segments of the citric acid cycle have been recognized.

An acetate is a salt formed by the combination of acetic acid with a base. "Acetate" also describes the conjugate base or ion typically found in aqueous solution and written with the chemical formula C
2H
3O−
2. The neutral molecules formed by the combination of the acetate ion and a positive ion are also commonly called "acetates". The simplest of these is hydrogen acetate with corresponding salts, esters, and the polyatomic anion CH
3CO−
2, or CH
3COO−
.
The iron–sulfur world hypothesis is a set of proposals for the origin of life and the early evolution of life advanced in a series of articles between 1988 and 1992 by Günter Wächtershäuser, a Munich patent lawyer with a degree in chemistry, who had been encouraged and supported by philosopher Karl R. Popper to publish his ideas. The hypothesis proposes that early life may have formed on the surface of iron sulfide minerals, hence the name. It was developed by retrodiction from extant biochemistry in conjunction with chemical experiments.
The term amphibolic is used to describe a biochemical pathway that involves both catabolism and anabolism. Catabolism is a degradative phase of metabolism in which large molecules are converted into smaller and simpler molecules, which involves two types of reactions. First, hydrolysis reactions, in which catabolism is the breaking apart of molecules into smaller molecules to release energy. Examples of catabolic reactions are digestion and cellular respiration, where sugars and fats are broken down for energy. Breaking down a protein into amino acids, or a triglyceride into fatty acids, or a disaccharide into monosaccharides are all hydrolysis or catabolic reactions. Second, oxidation reactions involve the removal of hydrogens and electrons from an organic molecule. Anabolism is the biosynthesis phase of metabolism in which smaller simple precursors are converted to large and complex molecules of the cell. Anabolism has two classes of reactions. The first are dehydration synthesis reactions; these involve the joining of smaller molecules together to form larger, more complex molecules. These include the formation of carbohydrates, proteins, lipids and nucleic acids. The second are reduction reactions, in which hydrogens and electrons are added to a molecule. Whenever that is done, molecules gain energy.

Biological carbon fixation or сarbon assimilation is the process by which inorganic carbon is converted to organic compounds by living organisms. The compounds are then used to store energy and as structure for other biomolecules. Carbon is primarily fixed through photosynthesis, but some organisms use a process called chemosynthesis in the absence of sunlight.
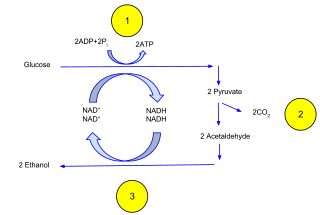
Ethanol fermentation, also called alcoholic fermentation, is a biological process which converts sugars such as glucose, fructose, and sucrose into cellular energy, producing ethanol and carbon dioxide as by-products. Because yeasts perform this conversion in the absence of oxygen, alcoholic fermentation is considered an anaerobic process. It also takes place in some species of fish where it provides energy when oxygen is scarce.

Sodium acetate, CH3COONa, also abbreviated NaOAc, is the sodium salt of acetic acid. This colorless deliquescent salt has a wide range of uses.
An acetogen is a microorganism that generates acetate (CH3COO−) as an end product of anaerobic respiration or fermentation. However, this term is usually employed in a narrower sense only to those bacteria and archaea that perform anaerobic respiration and carbon fixation simultaneously through the reductive acetyl coenzyme A (acetyl-CoA) pathway (also known as the Wood-Ljungdahl pathway). These genuine acetogens are also known as "homoacetogens" and they can produce acetyl-CoA (and from that, in most cases, acetate as the end product) from two molecules of carbon dioxide (CO2) and four molecules of molecular hydrogen (H2). This process is known as acetogenesis, and is different from acetate fermentation, although both occur in the absence of molecular oxygen (O2) and produce acetate. Although previously thought that only bacteria are acetogens, some archaea can be considered to be acetogens.
Acidogenesis is the second stage in the four stages of anaerobic digestion:

In biochemistry, mixed acid fermentation is the metabolic process by which a six-carbon sugar is converted into a complex and variable mixture of acids. It is an anaerobic (non-oxygen-requiring) fermentation reaction that is common in bacteria. It is characteristic for members of the Enterobacteriaceae, a large family of Gram-negative bacteria that includes E. coli.
Microbial metabolism is the means by which a microbe obtains the energy and nutrients it needs to live and reproduce. Microbes use many different types of metabolic strategies and species can often be differentiated from each other based on metabolic characteristics. The specific metabolic properties of a microbe are the major factors in determining that microbe's ecological niche, and often allow for that microbe to be useful in industrial processes or responsible for biogeochemical cycles.
In biology, syntrophy, syntrophism, or cross-feeding is the cooperative interaction between at least two microbial species to degrade a single substrate. This type of biological interaction typically involves the transfer of one or more metabolic intermediates between two or more metabolically diverse microbial species living in close proximity to each other. Thus, syntrophy can be considered an obligatory interdependency and a mutualistic metabolism between different microbial species, wherein the growth of one partner depends on the nutrients, growth factors, or substrates provided by the other(s).

Fermentation is a metabolic process that produces chemical changes in organic substances through the action of enzymes. In biochemistry, it is broadly defined as the extraction of energy from carbohydrates in the absence of oxygen. In food production, it may more broadly refer to any process in which the activity of microorganisms brings about a desirable change to a foodstuff or beverage. The science of fermentation is known as zymology.

The Wood–Ljungdahl pathway is a set of biochemical reactions used by some bacteria. It is also known as the reductive acetyl-coenzyme A (acetyl-CoA) pathway. This pathway enables these organisms to use hydrogen as an electron donor, and carbon dioxide as an electron acceptor and as a building block for biosynthesis.
In enzymology, carbon monoxide dehydrogenase (CODH) (EC 1.2.7.4) is an enzyme that catalyzes the chemical reaction
Fermentative hydrogen production is the fermentative conversion of organic substrates to H2. Hydrogen produced in this manner is often called biohydrogen. The conversion is effected by bacteria and protozoa, which employ enzymes. Fermentative hydrogen production is one of several anaerobic conversions.

Acetic acid, systematically named ethanoic acid, is an acidic, colourless liquid and organic compound with the chemical formula CH3COOH. Vinegar is at least 4% acetic acid by volume, making acetic acid the main component of vinegar apart from water. It has been used, as a component of vinegar, throughout history from at least the third century BC.
Acetobacterium is a genus of anaerobic, Gram-positive bacteria that belong to the Eubacteriaceae family. The type species of this genus is Acetobacterium woodii. The name, Acetobacterium, has originated because they are acetogens, predominantly making acetic acid as a byproduct of anaerobic metabolism. Most of the species reported in this genus are homoacetogens, i.e. solely producing acetic acid as their metabolic byproduct. They should not be confused with acetic acid bacteria which are aerobic, Gram-negative Alphaproteobacteria.
Hydrogenotrophs are organisms that are able to metabolize molecular hydrogen as a source of energy.

Acetyl-CoA synthase (ACS), not to be confused with acetyl-CoA synthetase or acetate-CoA ligase, is a nickel-containing enzyme involved in the metabolic processes of cells. Together with carbon monoxide dehydrogenase (CODH), it forms the bifunctional enzyme Acetyl-CoA Synthase/Carbon Monoxide Dehydrogenase (ACS/CODH) found in anaerobic microorganisms such as archaea and bacteria. The ACS/CODH enzyme works primarily through the Wood–Ljungdahl pathway which converts carbon dioxide to Acetyl-CoA. The recommended name for this enzyme is CO-methylating acetyl-CoA synthase.
References
- ↑ Angelidaki I, Karakashev D, Batstone DJ, Plugge CM, Stams AJ (2011). "16. Biomethanation and Its Potential". In Rosenzweig AC, Ragsdale SW (eds.). Methods in Enzymology. Methods in Methane Metabolism, Part A. Vol. 494. Academic Press. pp. 327–351. doi:10.1016/B978-0-12-385112-3.00016-0. ISBN 978-0-123-85112-3. PMID 21402222.
- ↑ Singleton P (2006). "Acetogenesis". Dictionary of microbiology and molecular biology (3rd ed.). Chichester: John Wiley. ISBN 978-0-470-03545-0.
- 1 2 3 Ragsdale SW, Pierce E (December 2008). "Acetogenesis and the Wood-Ljungdahl pathway of CO2 fixation". Biochimica et Biophysica Acta (BBA) – Proteins and Proteomics. 1784 (12): 1873–98. doi:10.1016/j.bbapap.2008.08.012. PMC 2646786 . PMID 18801467.
- ↑ Ragsdale SW (August 2006). "Metals and their scaffolds to promote difficult enzymatic reactions". Chemical Reviews. 106 (8): 3317–37. doi:10.1021/cr0503153. PMID 16895330.
- ↑ Schuchmann K, Müller V (July 2016). "Energetics and Application of Heterotrophy in Acetogenic Bacteria". Applied and Environmental Microbiology. 82 (14): 4056–69. Bibcode:2016ApEnM..82.4056S. doi:10.1128/AEM.00882-16. PMC 4959221 . PMID 27208103.