
Microfilaments, also called actin filaments, are protein filaments in the cytoplasm of eukaryotic cells that form part of the cytoskeleton. They are primarily composed of polymers of actin, but are modified by and interact with numerous other proteins in the cell. Microfilaments are usually about 7 nm in diameter and made up of two strands of actin. Microfilament functions include cytokinesis, amoeboid movement, cell motility, changes in cell shape, endocytosis and exocytosis, cell contractility, and mechanical stability. Microfilaments are flexible and relatively strong, resisting buckling by multi-piconewton compressive forces and filament fracture by nanonewton tensile forces. In inducing cell motility, one end of the actin filament elongates while the other end contracts, presumably by myosin II molecular motors. Additionally, they function as part of actomyosin-driven contractile molecular motors, wherein the thin filaments serve as tensile platforms for myosin's ATP-dependent pulling action in muscle contraction and pseudopod advancement. Microfilaments have a tough, flexible framework which helps the cell in movement.

Actin is a family of globular multi-functional proteins that form microfilaments in the cytoskeleton, and the thin filaments in muscle fibrils. It is found in essentially all eukaryotic cells, where it may be present at a concentration of over 100 μM; its mass is roughly 42 kDa, with a diameter of 4 to 7 nm.
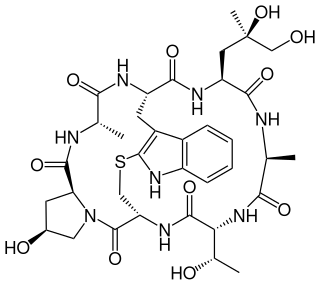
Phalloidin belongs to a class of toxins called phallotoxins, which are found in the death cap mushroom (Amanita phalloides). It is a rigid bicyclic heptapeptide that is lethal after a few days when injected into the bloodstream. The major symptom of phalloidin poisoning is acute hunger due to the destruction of liver cells. It functions by binding and stabilizing filamentous actin (F-actin) and effectively prevents the depolymerization of actin fibers. Due to its tight and selective binding to F-actin, derivatives of phalloidin containing fluorescent tags are used widely in microscopy to visualize F-actin in biomedical research.

Cytochalasin B, the name of which comes from the Greek cytos (cell) and chalasis (relaxation), is a cell-permeable mycotoxin. It was found that substoichiometric concentrations of cytochalasin B (CB) strongly inhibit network formation by actin filaments. Due to this, it is often used in cytological research. It inhibits cytoplasmic division by blocking the formation of contractile microfilaments. It inhibits cell movement and induces nuclear extrusion. Cytochalasin B shortens actin filaments by blocking monomer addition at the fast-growing end of polymers. Cytochalasin B inhibits glucose transport and platelet aggregation. It blocks adenosine-induced apoptotic body formation without affecting activation of endogenous ADP-ribosylation in leukemia HL-60 cells. It is also used in cloning through nuclear transfer. Here enucleated recipient cells are treated with cytochalasin B. Cytochalasin B makes the cytoplasm of the oocytes more fluid and makes it possible to aspirate the nuclear genome of the oocyte within a small vesicle of plasma membrane into a micro-needle. Thereby, the oocyte genome is removed from the oocyte, while preventing rupture of the plasma membrane.
Actin-binding proteins are proteins that bind to actin. This may mean ability to bind actin monomers, or polymers, or both.
Profilin is an actin-binding protein involved in the dynamic turnover and reconstruction of the actin cytoskeleton. It is found in most eukaryotic organisms. Profilin is important for spatially and temporally controlled growth of actin microfilaments, which is an essential process in cellular locomotion and cell shape changes. This restructuring of the actin cytoskeleton is essential for processes such as organ development, wound healing, and the hunting down of infectious intruders by cells of the immune system.
The lamellipodium is a cytoskeletal protein actin projection on the leading edge of the cell. It contains a quasi-two-dimensional actin mesh; the whole structure propels the cell across a substrate. Within the lamellipodia are ribs of actin called microspikes, which, when they spread beyond the lamellipodium frontier, are called filopodia. The lamellipodium is born of actin nucleation in the plasma membrane of the cell and is the primary area of actin incorporation or microfilament formation of the cell.
ADF/cofilin is a family of actin-binding proteins associated with the rapid depolymerization of actin microfilaments that give actin its characteristic dynamic instability. This dynamic instability is central to actin's role in muscle contraction, cell motility and transcription regulation.
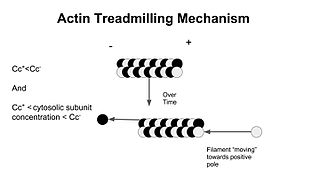
In molecular biology, treadmilling is a phenomenon observed within protein filaments of the cytoskeletons of many cells, especially in actin filaments and microtubules. It occurs when one end of a filament grows in length while the other end shrinks, resulting in a section of filament seemingly "moving" across a stratum or the cytosol. This is due to the constant removal of the protein subunits from these filaments at one end of the filament, while protein subunits are constantly added at the other end. Treadmilling was discovered by Wegner, who defined the thermodynamic and kinetic constraints. Wegner recognized that: “The equilibrium constant (K) for association of a monomer with a polymer is the same at both ends, since the addition of a monomer to each end leads to the same polymer.”; a simple reversible polymer can’t treadmill; ATP hydrolysis is required. GTP is hydrolyzed for microtubule treadmilling.

In biology, a protein filament is a long chain of protein monomers, such as those found in hair, muscle, or in flagella. Protein filaments form together to make the cytoskeleton of the cell. They are often bundled together to provide support, strength, and rigidity to the cell. When the filaments are packed up together, they are able to form three different cellular parts. The three major classes of protein filaments that make up the cytoskeleton include: actin filaments, microtubules and intermediate filaments.
Tropomodulin (TMOD) is a protein which binds and caps the minus end of actin, regulating the length of actin filaments in muscle and non-muscle cells.

Cortactin is a monomeric protein located in the cytoplasm of cells that can be activated by external stimuli to promote polymerization and rearrangement of the actin cytoskeleton, especially the actin cortex around the cellular periphery. It is present in all cell types. When activated, it will recruit Arp2/3 complex proteins to existing actin microfilaments, facilitating and stabilizing nucleation sites for actin branching. Cortactin is important in promoting lamellipodia formation, invadopodia formation, cell migration, and endocytosis.
CapZ, also known as CAPZ, CAZ1 and CAPPA1, is a capping protein that caps the barbed end of actin filaments in muscle cells.

Protein cordon-bleu is a protein that in humans is encoded by the COBL gene.

The Actin assembly-inducing protein (ActA) is a protein encoded and used by Listeria monocytogenes to propel itself through a mammalian host cell. ActA is a bacterial surface protein comprising a membrane-spanning region. In a mammalian cell the bacterial ActA interacts with the Arp2/3 complex and actin monomers to induce actin polymerization on the bacterial surface generating an actin comet tail. The gene encoding ActA is named actA or prtB.
Actin remodeling is a biochemical process in cells. In the actin remodeling of neurons, the protein actin is part of the process to change the shape and structure of dendritic spines. G-actin is the monomer form of actin, and is uniformly distributed throughout the axon and the dendrite. F-actin is the polymer form of actin, and its presence in dendritic spines is associated with their change in shape and structure. Actin plays a role in the formation of new spines as well as stabilizing spine volume increase. The changes that actin brings about lead to the formation of new synapses as well as increased cell communication.
mDia1 is a member of the protein family called the formins and is a Rho effector. It is the mouse version of the diaphanous homolog 1 of Drosophila. mDia1 localizes to cells' mitotic spindle and midbody, plays a role in stress fiber and filopodia formation, phagocytosis, activation of serum response factor, formation of adherens junctions, and it can act as a transcription factor. mDia1 accelerates actin nucleation and elongation by interacting with barbed ends of actin filaments. The gene encoding mDia1 is located on Chromosome 18 of Mus musculus and named Diap1.

Arp2/3 complex is a seven-subunit protein complex that plays a major role in the regulation of the actin cytoskeleton. It is a major component of the actin cytoskeleton and is found in most actin cytoskeleton-containing eukaryotic cells. Two of its subunits, the Actin-Related Proteins ARP2 and ARP3, closely resemble the structure of monomeric actin and serve as nucleation sites for new actin filaments. The complex binds to the sides of existing ("mother") filaments and initiates growth of a new ("daughter") filament at a distinctive 70-degree angle from the mother. Branched actin networks are created as a result of this nucleation of new filaments. The regulation of rearrangements of the actin cytoskeleton is important for processes like cell locomotion, phagocytosis, and intracellular motility of lipid vesicles.

In molecular biology, the cyclase-associated protein family (CAP) is a family of highly conserved actin-binding proteins present in a wide range of organisms including yeast, flies, plants, and mammals. CAPs are multifunctional proteins that contain several structural domains. CAP is involved in species-specific signalling pathways. In Drosophila, CAP functions in Hedgehog-mediated eye development and in establishing oocyte polarity. In Dictyostelium discoideum, CAP is involved in microfilament reorganisation near the plasma membrane in a PIP2-regulated manner and is required to perpetuate the cAMP relay signal to organise fruitbody formation. In plants, CAP is involved in plant signalling pathways required for co-ordinated organ expansion. In yeast, CAP is involved in adenylate cyclase activation, as well as in vesicle trafficking and endocytosis. In both yeast and mammals, CAPs appear to be involved in recycling G-actin monomers from ADF/cofilins for subsequent rounds of filament assembly. In mammals, there are two different CAPs that share 64% amino acid identity.
An actin nucleation core is a protein trimer with three actin monomers. It is called a nucleation core because it leads to the energetically favorable elongation reaction once a tetramer is formed from a trimer. Actin protein dimers and trimers are energetically unfavorable. Actin nucleators like the Arp2/3 complex of proteins from the formin family are most frequently involved in this process. Actin nucleation factors start the polymerization of actin within cells.















