Related Research Articles
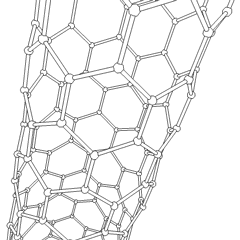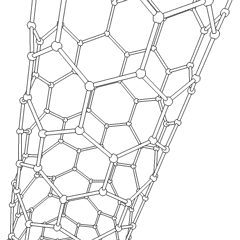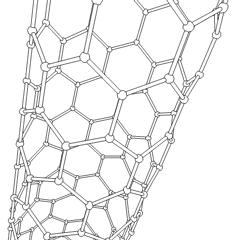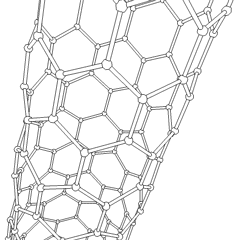
A carbon nanotube (CNT) is a tube made of carbon with a diameter in the nanometre range (nanoscale). They are one of the allotropes of carbon. Two broad classes of carbon nanotubes are recognized:
An elastic modulus is the unit of measurement of an object's or substance's resistance to being deformed elastically when a stress is applied to it.
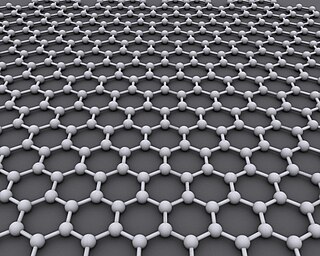
Graphene is a carbon allotrope consisting of a single layer of atoms arranged in a honeycomb planar nanostructure. The name "graphene" is derived from "graphite" and the suffix -ene, indicating the presence of double bonds within the carbon structure.
Specific modulus is a materials property consisting of the elastic modulus per mass density of a material. It is also known as the stiffness to weight ratio or specific stiffness. High specific modulus materials find wide application in aerospace applications where minimum structural weight is required. The dimensional analysis yields units of distance squared per time squared. The equation can be written as:

Nanocomposite is a multiphase solid material where one of the phases has one, two or three dimensions of less than 100 nanometers (nm) or structures having nano-scale repeat distances between the different phases that make up the material.
Methods have been devised to modify the yield strength, ductility, and toughness of both crystalline and amorphous materials. These strengthening mechanisms give engineers the ability to tailor the mechanical properties of materials to suit a variety of different applications. For example, the favorable properties of steel result from interstitial incorporation of carbon into the iron lattice. Brass, a binary alloy of copper and zinc, has superior mechanical properties compared to its constituent metals due to solution strengthening. Work hardening has also been used for centuries by blacksmiths to introduce dislocations into materials, increasing their yield strengths.

Nanocellulose is a term referring to a family of cellulosic materials that have at least one of their dimensions in the nanoscale. Examples of nanocellulosic materials are microfibrilated cellulose, cellulose nanofibers or cellulose nanocrystals. Nanocellulose may be obtained from natural cellulose fibers through a variety of production processes. This family of materials possesses interesting properties suitable for a wide range of potential applications.
Ultralight materials are solids with a density of less than 10 mg/cm3, including silica aerogels, carbon nanotube aerogels, aerographite, metallic foams, polymeric foams, and metallic microlattices. The density of air is about 1.275 mg/cm3, which means that the air in the pores contributes significantly to the density of these materials in atmospheric conditions. They can be classified by production method as aerogels, stochastic foams, and structured cellular materials.

A metallic microlattice is a synthetic porous metallic material consisting of an ultra-light metal foam. With a density as low as 0.99 mg/cm3 (0.00561 lb/ft3), it is one of the lightest structural materials known to science. It was developed by a team of scientists from California-based HRL Laboratories, in collaboration with researchers at University of California, Irvine and Caltech, and was first announced in November 2011. The prototype samples were made from a nickel-phosphorus alloy. In 2012, the microlattice prototype was declared one of 10 World-Changing Innovations by Popular Mechanics. Metallic microlattice technology has numerous potential applications in automotive and aeronautical engineering. A detailed comparative review study among other types of metallic lattice structures showed them to be beneficial for light-weighting purposes but expensive to manufacture.

Aerographite is a synthetic foam consisting of a porous interconnected network of tubular carbon. With a density of 180 g/m3 it is one of the lightest structural materials ever created. It was developed jointly by a team of researchers at the University of Kiel and the Technical University of Hamburg in Germany, and was first reported in a scientific journal in June 2012.

Freeze-casting, also frequently referred to as ice-templating, freeze casting, or freeze alignment, is a technique that exploits the highly anisotropic solidification behavior of a solvent in a well-dispersed solution or slurry to controllably template directionally porous ceramics, polymers, metals and their hybrids. By subjecting an aqueous solution or slurry to a directional temperature gradient, ice crystals will nucleate on one side and grow along the temperature gradient. The ice crystals will redistribute the dissolved substance and the suspended particles as they grow within the solution or slurry, effectively templating the ingredients that are distributed in the solution or slurry.
Reversibly assembled cellular composite materials (RCCM) are three-dimensional lattices of modular structures that can be partially disassembled to enable repairs or other modifications. Each cell incorporates structural material and a reversible interlock, allowing lattices of arbitrary size and shape. RCCM display three-dimensional symmetry derived from the geometry as linked.
Tube-based nanostructures are nanolattices made of connected tubes and exhibit nanoscale organization above the molecular level.
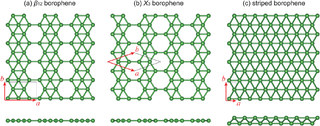
Borophene is a crystalline atomic monolayer of boron, i.e., it is a two-dimensional allotrope of boron and also known as boron sheet. First predicted by theory in the mid-1990s, different borophene structures were experimentally confirmed in 2015.
A two-dimensional semiconductor is a type of natural semiconductor with thicknesses on the atomic scale. Geim and Novoselov et al. initiated the field in 2004 when they reported a new semiconducting material graphene, a flat monolayer of carbon atoms arranged in a 2D honeycomb lattice. A 2D monolayer semiconductor is significant because it exhibits stronger piezoelectric coupling than traditionally employed bulk forms. This coupling could enable applications. One research focus is on designing nanoelectronic components by the use of graphene as electrical conductor, hexagonal boron nitride as electrical insulator, and a transition metal dichalcogenide as semiconductor.

Aerogels are a class of synthetic porous ultralight material derived from a gel, in which the liquid component for the gel has been replaced with a gas, without significant collapse of the gel structure. The result is a solid with extremely low density and extremely low thermal conductivity. Aerogels can be made from a variety of chemical compounds. Silica aerogels feel like fragile styrofoam to the touch, while some polymer-based aerogels feel like rigid foams.
Microscale structural metamaterials are synthetic structures that are aimed to yield specific desired mechanical advantages. These designs are often inspired by natural cellular materials such as plant and bone tissue which have superior mechanical efficiency due to their low weight to stiffness ratios.
A graphene morphology is any of the structures related to, and formed from, single sheets of graphene. 'Graphene' is typically used to refer to the crystalline monolayer of the naturally occurring material graphite. Due to quantum confinement of electrons within the material at these low dimensions, small differences in graphene morphology can greatly impact the physical and chemical properties of these materials. Commonly studied graphene morphologies include the monolayer sheets, bilayer sheets, graphene nanoribbons and other 3D structures formed from stacking of the monolayer sheets.
Biofoams are biological or biologically derived foams, making up lightweight and porous cellular solids. A relatively new term, its use in academia began in the 1980s in relation to the scum that formed on activated sludge plants.
Porous carbons (PCs) are versatile materials with a wide range of applications, including sensors, actuators, thermal insulation, and energy conversion. Some examples of PCs are graphene and carbon nanotube-based aerogel. Physical properties that make PCs unique are their low density, high conductivity, mechanical flexibility, and stability in extreme environments.
References
- 1 2 3 Guinness World Records 2018. Jim Pattison Group. 7 September 2017. p. 188. ISBN 9781910561713.
- ↑ "Ultra-light Aerogel Produced at a Zhejiang University Lab-Press Releases-Zhejiang University". Zju.edu.cn. 2013-03-19. Archived from the original on 2013-05-23. Retrieved 2013-06-12.
- ↑ Mecklenburg, M.; Schuchardt, A.; Mishra, Y. K.; Kaps, S. R.; Adelung, R.; Lotnyk, A.; Kienle, L.; Schulte, K. (2012). "Aerographite: Ultra Lightweight, Flexible Nanowall, Carbon Microtube Material with Outstanding Mechanical Performance". Advanced Materials. 24 (26): 3486–3490. Bibcode:2012AdM....24.3486M. doi:10.1002/adma.201200491. PMID 22688858. S2CID 2787227.
- ↑ Starr, Michelle (2013-03-25). "Graphene aerogel is the new world's lightest substance". Archived from the original on 2013-06-30. Retrieved 2013-09-06.
- 1 2 Hu, Han; Zhao, Zongbin; Wan, Wubo; Gogotsi, Yury; Qiu, Jieshan (2013). "Ultralight and Highly Compressible Graphene Aerogels". Advanced Materials. 25 (15): 2219–2223. Bibcode:2013AdM....25.2219H. doi:10.1002/adma.201204530. ISSN 1521-4095. PMID 23418081. S2CID 38156706.
- 1 2 3 Zhu, Cheng; Han, T. Yong-Jin; Duoss, Eric B.; Golobic, Alexandra M.; Kuntz, Joshua D.; Spadaccini, Christopher M.; Worsley, Marcus A. (2015-04-22). "Highly compressible 3D periodic graphene aerogel microlattices". Nature Communications. 6 (1): 6962. Bibcode:2015NatCo...6.6962Z. doi: 10.1038/ncomms7962 . ISSN 2041-1723. PMC 4421818 . PMID 25902277.
- 1 2 Worsley, Marcus A.; Kucheyev, Sergei O.; Mason, Harris E.; Merrill, Matthew D.; Mayer, Brian P.; Lewicki, James; Valdez, Carlos A.; Suss, Matthew E.; Stadermann, Michael; Pauzauskie, Peter J.; Satcher, Joe H. (2012-07-25). "Mechanically robust 3D graphene macroassembly with high surface area". Chemical Communications. 48 (67): 8428–8430. doi:10.1039/C2CC33979J. ISSN 1364-548X. PMID 22797515.
- 1 2 3 Qin, Zhao; Jung, Gang Seob; Kang, Min Jeong; Buehler, Markus J. (2017-01-01). "The mechanics and design of a lightweight three-dimensional graphene assembly". Science Advances. 3 (1): e1601536. Bibcode:2017SciA....3E1536Q. doi: 10.1126/sciadv.1601536 . ISSN 2375-2548. PMC 5218516 . PMID 28070559.
- ↑ Lei, Jincheng; Liu, Zishun (2018-04-01). "The structural and mechanical properties of graphene aerogels based on Schwarz-surface-like graphene models". Carbon. 130: 741–748. doi:10.1016/j.carbon.2018.01.061. ISSN 0008-6223.
- ↑ Wu, Yingpeng; Yi, Ningbo; Huang, Lu; Zhang, Tengfei; Fang, Shaoli; Chang, Huicong; Li, Na; Oh, Jiyoung; Lee, Jae Ah; Kozlov, Mikhail; Chipara, Alin C. (2015-01-20). "Three-dimensionally bonded spongy graphene material with super compressive elasticity and near-zero Poisson's ratio". Nature Communications. 6 (1): 6141. Bibcode:2015NatCo...6.6141W. doi: 10.1038/ncomms7141 . ISSN 2041-1723. PMID 25601131.
- ↑ Chen, Bo; Ma, Qinglang; Tan, Chaoliang; Lim, Teik-Thye; Huang, Ling; Zhang, Hua (2015-03-23). "Carbon-Based Sorbents with Three-Dimensional Architectures for Water Remediation". Small . 11 (27). Wiley-VCH: 3319–3336. doi:10.1002/smll.201403729. eISSN 1613-6829. ISSN 1613-6810. PMID 25808922.