Related Research Articles

Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential difference and identifiable chemical change. These reactions involve electrons moving via an electronically-conducting phase between electrodes separated by an ionically conducting and electronically insulating electrolyte.
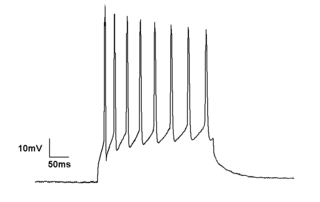
Electrophysiology is the branch of physiology that studies the electrical properties of biological cells and tissues. It involves measurements of voltage changes or electric current or manipulations on a wide variety of scales from single ion channel proteins to whole organs like the heart. In neuroscience, it includes measurements of the electrical activity of neurons, and, in particular, action potential activity. Recordings of large-scale electric signals from the nervous system, such as electroencephalography, may also be referred to as electrophysiological recordings. They are useful for electrodiagnosis and monitoring.
A biosensor is an analytical device, used for the detection of a chemical substance, that combines a biological component with a physicochemical detector. The sensitive biological element, e.g. tissue, microorganisms, organelles, cell receptors, enzymes, antibodies, nucleic acids, etc., is a biologically derived material or biomimetic component that interacts with, binds with, or recognizes the analyte under study. The biologically sensitive elements can also be created by biological engineering. The transducer or the detector element, which transforms one signal into another one, works in a physicochemical way: optical, piezoelectric, electrochemical, electrochemiluminescence etc., resulting from the interaction of the analyte with the biological element, to easily measure and quantify. The biosensor reader device connects with the associated electronics or signal processors that are primarily responsible for the display of the results in a user-friendly way. This sometimes accounts for the most expensive part of the sensor device, however it is possible to generate a user friendly display that includes transducer and sensitive element. The readers are usually custom-designed and manufactured to suit the different working principles of biosensors.

In electrochemistry, cyclic voltammetry (CV) is a type of potentiodynamic measurement. In a cyclic voltammetry experiment, the working electrode potential is ramped linearly versus time. Unlike in linear sweep voltammetry, after the set potential is reached in a CV experiment, the working electrode's potential is ramped in the opposite direction to return to the initial potential. These cycles of ramps in potential may be repeated as many times as needed. The current at the working electrode is plotted versus the applied voltage to give the cyclic voltammogram trace. Cyclic voltammetry is generally used to study the electrochemical properties of an analyte in solution or of a molecule that is adsorbed onto the electrode.
Amperometric titration refers to a class of titrations in which the equivalence point is determined through measurement of the electric current produced by the titration reaction. It is a form of quantitative analysis.

Voltammetry is a category of electroanalytical methods used in analytical chemistry and various industrial processes. In voltammetry, information about an analyte is obtained by measuring the current as the potential is varied. The analytical data for a voltammetric experiment comes in the form of a voltammogram, which plots the current produced by the analyte versus the potential of the working electrode.
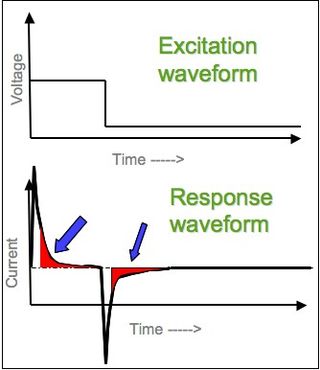
In electrochemistry, chronoamperometry is an analytical technique in which the electric potential of the working electrode is stepped and the resulting current from faradaic processes occurring at the electrode is monitored as a function of time. The functional relationship between current response and time is measured after applying single or double potential step to the working electrode of the electrochemical system. Limited information about the identity of the electrolyzed species can be obtained from the ratio of the peak oxidation current versus the peak reduction current. However, as with all pulsed techniques, chronoamperometry generates high charging currents, which decay exponentially with time as any RC circuit. The Faradaic current - which is due to electron transfer events and is most often the current component of interest - decays as described in the Cottrell equation. In most electrochemical cells, this decay is much slower than the charging decay-cells with no supporting electrolyte are notable exceptions. Most commonly a three-electrode system is used. Since the current is integrated over relatively longer time intervals, chronoamperometry gives a better signal-to-noise ratio in comparison to other amperometric techniques.
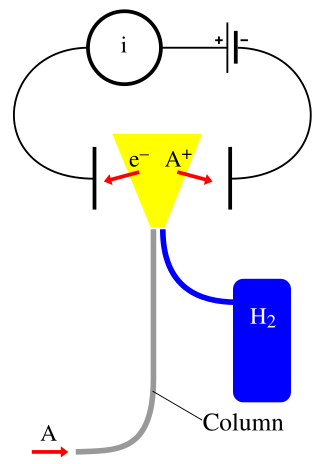
A flame ionization detector (FID) is a scientific instrument that measures analytes in a gas stream. It is frequently used as a detector in gas chromatography. The measurement of ions per unit time makes this a mass sensitive instrument. Standalone FIDs can also be used in applications such as landfill gas monitoring, fugitive emissions monitoring and internal combustion engine emissions measurement in stationary or portable instruments.
In electrochemistry, overpotential is the potential difference (voltage) between a half-reaction's thermodynamically determined reduction potential and the potential at which the redox event is experimentally observed. The term is directly related to a cell's voltage efficiency. In an electrolytic cell the existence of overpotential implies that the cell requires more energy than thermodynamically expected to drive a reaction. In a galvanic cell the existence of overpotential means less energy is recovered than thermodynamics predicts. In each case the extra/missing energy is lost as heat. The quantity of overpotential is specific to each cell design and varies across cells and operational conditions, even for the same reaction. Overpotential is experimentally determined by measuring the potential at which a given current density is achieved.
A potentiometric sensor is a type of chemical sensor that may be used to determine the analytical concentration of some components of the analyte gas or solution. These sensors measure the electrical potential of an electrode when no current is present.
In analytical chemistry, potentiometric titration is a technique similar to direct titration of a redox reaction. It is a useful means of characterizing an acid. No indicator is used; instead the electric potential is measured across the analyte, typically an electrolyte solution. To do this, two electrodes are used, an indicator electrode and a reference electrode. Reference electrodes generally used are hydrogen electrodes, calomel electrodes, and silver chloride electrodes. The indicator electrode forms an electrochemical half-cell with the interested ions in the test solution. The reference electrode forms the other half-cell.
Electroanalytical methods are a class of techniques in analytical chemistry which study an analyte by measuring the potential (volts) and/or current (amperes) in an electrochemical cell containing the analyte. These methods can be broken down into several categories depending on which aspects of the cell are controlled and which are measured. The three main categories are potentiometry, amperometry, coulometry.
Electrochemical gas sensors are gas detectors that measure the concentration of a target gas by oxidizing or reducing the target gas at an electrode and measuring the resulting current.
A biotransducer is the recognition-transduction component of a biosensor system. It consists of two intimately coupled parts; a bio-recognition layer and a physicochemical transducer, which acting together converts a biochemical signal to an electronic or optical signal. The bio-recognition layer typically contains an enzyme or another binding protein such as antibody. However, oligonucleotide sequences, sub-cellular fragments such as organelles and receptor carrying fragments, single whole cells, small numbers of cells on synthetic scaffolds, or thin slices of animal or plant tissues, may also comprise the bio-recognition layer. It gives the biosensor selectivity and specificity. The physicochemical transducer is typically in intimate and controlled contact with the recognition layer. As a result of the presence and biochemical action of the analyte, a physico-chemical change is produced within the biorecognition layer that is measured by the physicochemical transducer producing a signal that is proportionate to the concentration of the analyte. The physicochemical transducer may be electrochemical, optical, electronic, gravimetric, pyroelectric or piezoelectric. Based on the type of biotransducer, biosensors can be classified as shown to the right.

Fast-scan cyclic voltammetry (FSCV) is cyclic voltammetry with a very high scan rate (up to 1×106 V·s−1). Application of high scan rate allows rapid acquisition of a voltammogram within several milliseconds and ensures high temporal resolution of this electroanalytical technique. An acquisition rate of 10 Hz is routinely employed.

A field-effect transistor-based biosensor, also known as a biosensor field-effect transistor, field-effect biosensor (FEB), or biosensor MOSFET, is a field-effect transistor that is gated by changes in the surface potential induced by the binding of molecules. When charged molecules, such as biomolecules, bind to the FET gate, which is usually a dielectric material, they can change the charge distribution of the underlying semiconductor material resulting in a change in conductance of the FET channel. A Bio-FET consists of two main compartments: one is the biological recognition element and the other is the field-effect transistor. The BioFET structure is largely based on the ion-sensitive field-effect transistor (ISFET), a type of metal–oxide–semiconductor field-effect transistor (MOSFET) where the metal gate is replaced by an ion-sensitive membrane, electrolyte solution, and reference electrode.

Electrochemical stripping analysis is a set of analytical chemistry methods based on voltammetry or potentiometry that are used for quantitative determination of ions in solution. Stripping voltammetry have been employed for analysis of organic molecules as well as metal ions. Carbon paste, glassy carbon paste, and glassy carbon electrodes when modified are termed as chemically modified electrodes and have been employed for the analysis of organic and inorganic compounds.

Aptamers, single-stranded RNA and DNA sequences, bind to an analyte and change their conformation. They function as nucleic acids selectively binding molecules such as proteins, bacteria cells, metal ions, etc. Aptamers can be developed to have precise specificity to bind to a desired target. Aptamers change conformation upon binding, altering the electrochemical properties which can be measured. The Systematic Evolution of Ligands by Exponential Enrichment (SELEX) process generates aptamers. Electrochemical aptamer-based (E-AB) biosensors is a device that takes advantage of the electrochemical and biological properties of aptamers to take real time, in vivo measurements.
Single-Entity Electrochemistry (SEE) refers to the electroanalysis of an individual unit of interest. A unique feature of SEE is that it unifies multiple different branches of electrochemistry. Single-Entity Electrochemistry pushes the bounds of the field as it can measure entities on a scale of 100 microns to angstroms. Single-Entity Electrochemistry is important because it gives the ability to view how a single molecule, or cell, or "thing" affects the bulk response, and thus the chemistry that might have gone unknown otherwise. The ability to monitor the movement of one electron or ion from one unit to another is valuable, as many vital reactions and mechanisms undergo this process. Electrochemistry is well suited for this measurement due to its incredible sensitivity. Single-Entity Electrochemistry can be used to investigate nanoparticles, wires, vesicles, nanobubbles, nanotubes, cells, and viruses, and other small molecules and ions. Single-entity electrochemistry has been successfully used to determine the size distribution of particles as well as the number of particles present inside a vesicle or other similar structures
Screen-printed electrodes (SPEs) are electrochemical measurement devices that are manufactured by printing different types of ink on plastic or ceramic substrates, allowing quick in-situ analysis with high reproducibility, sensitivity and accuracy. The composition of the different inks used in the manufacture of the electrode determines its selectivity and sensitivity. This fact allows the analyst to design the most optimal device according to its purpose.
References
- ↑ D. C. Johnson and W.R. LaCourse, Analytical Chemistry, 62 (1990), 589A-97A
- ↑ Kissinger PT, Hart JB, Adams RN (May 1973). "Voltammetry in brain tissue--a new neurophysiological measurement". Brain Research. 55 (1): 209–13. doi:10.1016/0006-8993(73)90503-9. PMID 4145914.
- ↑ Gonon F, Cespuglio R, Ponchon JL, et al. (April 1978). "In vivo continuous electrochemical determination of dopamine release in rat neostriatum". Comptes Rendus de l'Académie des Sciences, Série D (in French). 286 (16): 1203–6. PMID 96981.
- ↑ Leszczyszyn DJ, Jankowski JA, Viveros OH, Diliberto EJ, Near JA, Wightman RM (September 1990). "Nicotinic receptor-mediated catecholamine secretion from individual chromaffin cells. Chemical evidence for exocytosis". The Journal of Biological Chemistry. 265 (25): 14736–7. doi: 10.1016/S0021-9258(18)77173-1 . PMID 2394692.
- ↑ Wightman RM, Jankowski JA, Kennedy RT, et al. (December 1991). "Temporally resolved catecholamine spikes correspond to single vesicle release from individual chromaffin cells". Proceedings of the National Academy of Sciences of the United States of America. 88 (23): 10754–8. Bibcode:1991PNAS...8810754W. doi: 10.1073/pnas.88.23.10754 . PMC 53009 . PMID 1961743.
- ↑ Settle, F. (Ed.). (1997). Handbook of Instrumental Techniques for Analytical Chemistry (1 ed.). Prentice Hall.
- ↑ Mosharov EV, Sulzer D (September 2005). "Analysis of exocytotic events recorded by amperometry". Nature Methods. 2 (9): 651–8. doi:10.1038/nmeth782. PMID 16118635. S2CID 15489257.