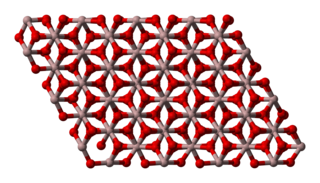
Aluminium oxide (or Aluminium(III) oxide) is a chemical compound of aluminium and oxygen with the chemical formula Al2O3. It is the most commonly occurring of several aluminium oxides, and specifically identified as aluminium oxide. It is commonly called alumina and may also be called aloxide, aloxite, or alundum in various forms and applications. It occurs naturally in its crystalline polymorphic phase α-Al2O3 as the mineral corundum, varieties of which form the precious gemstones ruby and sapphire. Al2O3 is significant in its use to produce aluminium metal, as an abrasive owing to its hardness, and as a refractory material owing to its high melting point.

Anodizing is an electrolytic passivation process used to increase the thickness of the natural oxide layer on the surface of metal parts.
A "photoelectrochemical cell" is one of two distinct classes of device. The first produces electrical energy similarly to a dye-sensitized photovoltaic cell, which meets the standard definition of a photovoltaic cell. The second is a photoelectrolytic cell, that is, a device which uses light incident on a photosensitizer, semiconductor, or aqueous metal immersed in an electrolytic solution to directly cause a chemical reaction, for example to produce hydrogen via the electrolysis of water.
Nanopillars is an emerging technology within the field of nanostructures. Nanopillars are pillar shaped nanostructures approximately 10 nanometers in diameter that can be grouped together in lattice like arrays. They are a type of metamaterial, which means that nanopillars get their attributes from being grouped into artificially designed structures and not their natural properties. Nanopillars set themselves apart from other nanostructures due to their unique shape. Each nanopillar has a pillar shape at the bottom and a tapered pointy end on top. This shape in combination with nanopillars' ability to be grouped together exhibits many useful properties. Nanopillars have many applications including efficient solar panels, high resolution analysis, and antibacterial surfaces.

Plasma electrolytic oxidation (PEO), also known as electrolytic plasma oxidation (EPO) or microarc oxidation (MAO), is an electrochemical surface treatment process for generating oxide coatings on metals. It is similar to anodizing, but it employs higher potentials, so that discharges occur and the resulting plasma modifies the structure of the oxide layer. This process can be used to grow thick, largely crystalline, oxide coatings on metals such as aluminium, magnesium and titanium. Because they can present high hardness and a continuous barrier, these coatings can offer protection against wear, corrosion or heat as well as electrical insulation.

Nanofoams are a class of nanostructured, porous materials (foams) containing a significant population of pores with diameters less than 100 nm. Aerogels are one example of nanofoam.

Nanoporous materials consist of a regular organic or inorganic bulk phase in which a porous structure is present. Nanoporous materials exhibit pore diameters that are most appropriately quantified using units of nanometers. The diameter of pores in nanoporous materials is thus typically 100 nanometers or smaller. Pores may be open or closed, and pore connectivity and void fraction vary considerably, as with other porous materials. Open pores are pores that connect to the surface of the material whereas closed pores are pockets of void space within a bulk material. Open pores are useful for molecular separation techniques, adsorption, and catalysis studies. Closed pores are mainly used in thermal insulators and for structural applications.
Aluminium–air batteries produce electricity from the reaction of oxygen in the air with aluminium. They have one of the highest energy densities of all batteries, but they are not widely used because of problems with high anode cost and byproduct removal when using traditional electrolytes. This has restricted their use to mainly military applications. However, an electric vehicle with aluminium batteries has the potential for up to eight times the range of a lithium-ion battery with a significantly lower total weight.

Nanobatteries are fabricated batteries employing technology at the nanoscale, particles that measure less than 100 nanometers or 10−7 meters. These batteries may be nano in size or may use nanotechnology in a macro scale battery. Nanoscale batteries can be combined to function as a macrobattery such as within a nanopore battery.

Aluminium smelting is the process of extracting aluminium from its oxide, alumina, generally by the Hall-Héroult process. Alumina is extracted from the ore bauxite by means of the Bayer process at an alumina refinery.
A nanowire battery uses nanowires to increase the surface area of one or both of its electrodes, which improves the capacity of the battery. Some designs, variations of the lithium-ion battery have been announced, although none are commercially available. All of the concepts replace the traditional graphite anode and could improve battery performance. Each type of nanowire battery has specific advantages and disadvantages, but a challenge common to all of them is their fragility.
Nanoarchitectures for lithium-ion batteries are attempts to employ nanotechnology to improve the design of lithium-ion batteries. Research in lithium-ion batteries focuses on improving energy density, power density, safety, durability and cost.

Local oxidation nanolithography (LON) is a tip-based nanofabrication method. It is based on the spatial confinement on an oxidation reaction under the sharp tip of an atomic force microscope.
The lithium–air battery (Li–air) is a metal–air electrochemical cell or battery chemistry that uses oxidation of lithium at the anode and reduction of oxygen at the cathode to induce a current flow.
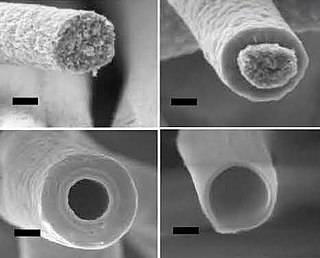
Silicon nanotubes are nanoparticles which create a tube-like structure from silicon atoms. As with silicon nanowires, they are technologically important due to their unusual physical properties, which differ fundamentally to those of bulk silicon. The first reports on silicon nanotubes appeared around the year 2000.
Carbon nanotube supported catalyst is a novel supported catalyst, using carbon nanotubes as the support instead of the conventional alumina or silicon support. The exceptional physical properties of carbon nanotubes (CNTs) such as large specific surface areas, excellent electron conductivity incorporated with the good chemical inertness, and relatively high oxidation stability makes it a promising support material for heterogeneous catalysis.
Aluminium-ion batteries are a class of rechargeable battery in which aluminium ions serve as charge carriers. Aluminium can exchange three electrons per ion. This means that insertion of one Al3+ is equivalent to three Li+ ions. Thus, since the ionic radii of Al3+ (0.54 Å) and Li+ (0.76 Å) are similar, significantly higher numbers of electrons and Al3+ ions can be accepted by cathodes with little damage. Al has 50 times (23.5 megawatt-hours m-3) the energy density of Li and is even higher than coal.

Nanobottles are hollow bottle-shaped particle with the maximal dimension smaller or comparable with 1 micrometer. They can be used for storing and distributing various chemical compounds, for example, inside the human body.

David Zitoun is an Israeli chemist and materials scientist.

Lynden A. Archer is a chemical engineer, Joseph Silbert Dean of Engineering, David Croll Director of the Energy Systems Institute, and professor of chemical engineering at Cornell University. He became a fellow of the American Physical Society in 2007 and was elected into the National Academy of Engineering in 2018. Archer's research covers polymer and hybrid materials and finds applications in energy storage technologies. His h-index is 92 by Google Scholar.












