Related Research Articles

AstraZeneca plc (AZ) is a British-Swedish multinational pharmaceutical and biotechnology company with its headquarters at the Cambridge Biomedical Campus in Cambridge, England. It has a portfolio of products for major diseases in areas including oncology, cardiovascular, gastrointestinal, infection, neuroscience, respiratory, and inflammation. It was involved in developing the Oxford–AstraZeneca COVID-19 vaccine.

An exotoxin is a toxin secreted by bacteria. An exotoxin can cause damage to the host by destroying cells or disrupting normal cellular metabolism. They are highly potent and can cause major damage to the host. Exotoxins may be secreted, or, similar to endotoxins, may be released during lysis of the cell. Gram negative pathogens may secrete outer membrane vesicles containing lipopolysaccharide endotoxin and some virulence proteins in the bounding membrane along with some other toxins as intra-vesicular contents, thus adding a previously unforeseen dimension to the well-known eukaryote process of membrane vesicle trafficking, which is quite active at the host–pathogen interface.
An immunotoxin is an artificial protein consisting of a targeting portion linked to a toxin. When the protein binds to that cell, it is taken in through endocytosis, and the toxin kills the cell. They are used for the treatment of some kinds of cancer and a few viral infections.

Hairy cell leukemia is an uncommon hematological malignancy characterized by an accumulation of abnormal B lymphocytes. It is usually classified as a subtype of chronic lymphocytic leukemia (CLL). Hairy cell leukemia makes up about 2% of all leukemias, with fewer than 2,000 new cases diagnosed annually in North America and Western Europe combined.
This is a list of terms related to oncology. The original source for this list was the US National Cancer Institute's public domain Dictionary of Cancer Terms.

MedImmune, LLC was a wholly owned subsidiary of AstraZeneca before February 14, 2019, when it was announced that the MedImmune name and branding would be discontinued in favor of AstraZeneca.
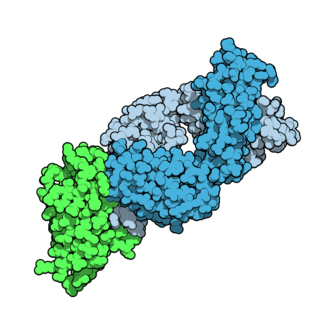
Tremelimumab, sold under the brand name Imjudo, is a fully human monoclonal antibody used for the treatment of hepatocellular carcinoma. Tremelimumab is designed to attach to and block CTLA-4, a protein that controls the activity of T cells, which are part of the immune system.
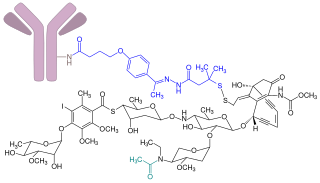
Inotuzumab ozogamicin, sold under the brand name Besponsa, is an antibody-drug conjugate medication used to treat relapsed or refractory B-cell precursor acute lymphoblastic leukemia. It is administered by intravenous infusion.

CD22, or cluster of differentiation-22, is a molecule belonging to the SIGLEC family of lectins. It is found on the surface of mature B cells and to a lesser extent on some immature B cells. Generally speaking, CD22 is a regulatory molecule that prevents the overactivation of the immune system and the development of autoimmune diseases.
Epratuzumab is a humanized monoclonal antibody. Potential uses may be found in oncology and in treatment of inflammatory autoimmune disorders, such as systemic lupus erythematosus (SLE).

Ira Pastan is an American scientist at the National Cancer Institute. He is a member of the National Academy of Sciences, a Fellow of the AAAS and the American Society of Microbiology. In 2009, he was awarded the prestigious International Antonio Feltrinelli Prize for Medicine. His wife, Linda Pastan, was an American poet.

Cambridge Antibody Technology Group Plc, was a biotechnology company headquartered in Cambridge, England, United Kingdom. Its core focus was on antibody therapeutics, primarily using Phage Display and Ribosome Display technology.
The Recombinant Immunotoxin Collaborative Group (RICG) is a group of scientists specialising in immunology, biochemistry and molecular biology from the United Kingdom and Italy. The group is working toward the development of genetically engineered immunotoxins made from monoclonal antibody fragments genetically fused to either saporin or Pseudomonas exotoxin (PE) for the treatment of human hematological malignancies such as leukaemia, lymphoma and multiple myeloma.
Gene expression profiling has revealed that diffuse large B-cell lymphoma (DLBCL) is composed of at least 3 different sub-groups, each having distinct oncogenic mechanisms that respond to therapies in different ways. Germinal Center B-Cell like (GCB) DLBCLs appear to arise from normal germinal center B cells, while Activated B-cell like (ABC) DLBCLs are thought to arise from postgerminal center B cells that are arrested during plasmacytic differentiation. The differences in gene expression between GCB DLBCL and ABC DLBCL are as vast as the differences between distinct types of leukemia, but these conditions have historically been grouped together and treated as the same disease.
Moxetumomab pasudotox, sold under the brand name Lumoxiti, is an anti-CD22 immunotoxin medication for the treatment of adults with relapsed or refractory hairy cell leukemia (HCL) who have received at least two prior systemic therapies, including treatment with a purine nucleoside analog. Moxetumomab pasudotox is a CD22-directed cytotoxin and is the first of this type of treatment for adults with HCL. The drug consists of the binding fragment (Fv) of an anti-CD22 antibody fused to a toxin called PE38. This toxin is a 38 kDa fragment of Pseudomonas exotoxin A.

Tralokinumab sold under the brand names Adtralza (EU/UK) and Adbry (US) among others, is a human monoclonal antibody used for the treatment of atopic dermatitis. Tralokinumab targets the cytokine interleukin 13.
Resimmune or A-dmDT390-bisFv(UCHT1) is an experimental drug — an anti-T cell immunotoxin — that is being investigated for the treatment of T cell blood cancers such as cutaneous T cell lymphoma (CTCL). It was developed by Doctors Neville, Woo, and Liu while at the National Institutes of Health (NIH) and is under exclusive license to Angimmune, LLC. The therapy has potential applications for lymphomas and T cell driven autoimmune diseases, including multiple sclerosis, and graft-versus-host disease following stem cell or bone marrow transplant.

PD-1 inhibitors and PD-L1 inhibitors are a group of checkpoint inhibitor anticancer drugs that block the activity of PD-1 and PDL1 immune checkpoint proteins present on the surface of cells. Immune checkpoint inhibitors are emerging as a front-line treatment for several types of cancer.

Camidanlumab tesirine is an antibody-drug conjugate (ADC) composed of a human antibody that binds to the protein CD25, conjugated to a pyrrolobenzodiazepine dimer toxin. The experimental drug, developed by ADC Therapeutics is being tested in clinical trials for the treatment of B-cell Hodgkin's lymphoma (HL) and non-Hodgkin lymphoma (NHL), and for the treatment of B-cell acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML).

Nirali N. Shah is an American physician-scientist and pediatric hematologist-oncologist, serving as head of the hematologic malignancies section of the pediatric oncology branch at the National Cancer Institute. She researches the translation of immunotherapeutic approaches to treat high-risk hematologic malignancies in children, adolescents and young adults.
References
- ↑ m. d, Robert Kreitman (7 October 2015). "Retreatment Protocol for BL22 Immunotherapy in Relapsed or Refractory Hairy Cell Leukemia".
- ↑ "Cambridge Antibody Technology Group PLC (CATG) Acquires Oncology Product Candidates From Genencor International, Inc. (GCOR)". 1 Nov 2005. Archived from the original on 1 August 2009. Retrieved 20 May 2010.
- 1 2 "Cambridge Antibody Technology Acquires Oncology Product Candidates from Genencor" (Press release). 1 Nov 2005.
- ↑ "Scientists Report Complete Remissions in Early Leukemia Trial".
- 1 2 http://www.cambridgeantibody.com/__data/assets/pdf_file/10857/CAT-3888,_CAT-8015_and_CAT-5001_Nov06.pdf Archived 2007-02-27 at the Wayback Machine CAT URL Redirects to Medimmune home page
- ↑ "Clinical Trials at NIH: Health Care Professionals: Investigator Profiles: Robert J. Kreitman, M.D." Archived from the original on 2011-10-15. Retrieved 2011-12-16.
- ↑ "MedImmune". Archived from the original on 2011-07-14. Retrieved 2010-11-25.
- ↑ "CAT-3888 MedImmune discontinued, USA (hairy cell leukemia)." 28 January 2008. R & D Focus Drug News. IMSworld Publications Ltd.
- ↑ "Phase I trial of recombinant immunotoxin CAT-8015 (HA22) in multiply relapsed hairy cell leukemia. -- Kreitman et al. 28 (15): 6523 -- ASCO Meeting Abstracts". Archived from the original on 2012-07-10. Retrieved 2011-12-16.
- ↑ "Chronic Lymphocytic Leukemia (CLL) Clinical Trial: A Phase 1-2 Study of CAT-8015 in Adult…". Archived from the original on 2012-07-11. Retrieved 2011-12-16.
- ↑ "CCR Clinical Trials at NIH: Patients and General Public: Clinical Trials Search at NIH". Archived from the original on 2011-12-11. Retrieved 2011-12-16.
- ↑ "CCR Clinical Trials at NIH: Patients and General Public: Clinical Trials Search at NIH". Archived from the original on 2011-12-11. Retrieved 2011-12-16.
- ↑ "America's Local Small Business Directory - Manta". Archived from the original on 2021-10-31. Retrieved 2010-05-20.
- ↑ "Media Centre - AstraZeneca".
- ↑ AstraZeneca Successfully Completes Acquisition of MedImmune Archived January 13, 2010, at the Wayback Machine
- ↑ AstraZeneca Presents its Global Biologics Organisation, MedImmune, at 2007 Analyst and Investor R&D Day
- ↑ "Mesothelioma Treatment product candidate SS1P renamed CAT-5001".