
Immunosuppressive drugs or immunosuppressive agents or antirejection medications are drugs that inhibit or prevent activity of the immune system.

Cancer immunotherapy is the artificial stimulation of the immune system to treat cancer, improving on the system's natural ability to fight cancer. It is an application of the fundamental research of cancer immunology and a growing subspecialty of oncology. It exploits the fact that cancer cells often have tumor antigens, molecules on their surface that can be detected by the antibody proteins of the immune system, binding to them. The tumor antigens are often proteins or other macromolecules. Normal antibodies bind to external pathogens, but the modified immunotherapy antibodies bind to the tumor antigens marking and identifying the cancer cells for the immune system to inhibit or kill.
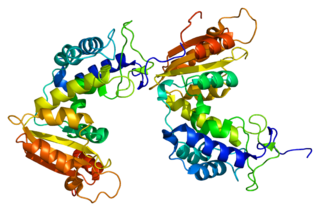
CD38 (cluster of differentiation 38), also known as cyclic ADP ribose hydrolase is a glycoprotein found on the surface of many immune cells (white blood cells), including CD4+, CD8+, B lymphocytes and natural killer cells. CD38 also functions in cell adhesion, signal transduction and calcium signaling.

The antibody-dependent cellular cytotoxicity (ADCC), also referred to as antibody-dependent cell-mediated cytotoxicity, is a mechanism of cell-mediated immune defense whereby an effector cell of the immune system actively lyses a target cell, whose membrane-surface antigens have been bound by specific antibodies. It is one of the mechanisms through which antibodies, as part of the humoral immune response, can act to limit and contain infection.
Humanized antibodies are antibodies from non-human species whose protein sequences have been modified to increase their similarity to antibody variants produced naturally in humans. The process of "humanization" is usually applied to monoclonal antibodies developed for administration to humans. Humanization can be necessary when the process of developing a specific antibody involves generation in a non-human immune system. The protein sequences of antibodies produced in this way are partially distinct from homologous antibodies occurring naturally in humans, and are therefore potentially immunogenic when administered to human patients. There are other types of antibodies developed. The International Nonproprietary Names of humanized antibodies end in -zumab, as in omalizumab.
The nomenclature of monoclonal antibodies is a naming scheme for assigning generic, or nonproprietary, names to monoclonal antibodies. An antibody is a protein that is produced in B cells and used by the immune system of humans and other vertebrate animals to identify a specific foreign object like a bacterium or a virus. Monoclonal antibodies are those that were produced in identical cells, often artificially, and so share the same target object. They have a wide range of applications including medical uses.

Monoclonal antibody therapy is a form of immunotherapy that uses monoclonal antibodies (mAb) to bind monospecifically to certain cells or proteins. The objective is that this treatment will stimulate the patient's immune system to attack those cells. Alternatively, in radioimmunotherapy a radioactive dose localizes a target cell line, delivering lethal chemical doses. More recently antibodies have been used to bind to molecules involved in T-cell regulation to remove inhibitory pathways that block T-cell responses. This is known as immune checkpoint therapy.

Programmed cell death protein 1, also known as PD-1 and CD279, is a protein on the surface of cells that has a role in regulating the immune system's response to the cells of the human body by down-regulating the immune system and promoting self-tolerance by suppressing T cell inflammatory activity. This prevents autoimmune diseases, but it can also prevent the immune system from killing cancer cells.

Lymphocyte-activation gene 3, also known as LAG-3, is a protein which in humans is encoded by the LAG3 gene. LAG3, which was discovered in 1990 and was designated CD223 after the Seventh Human Leucocyte Differentiation Antigen Workshop in 2000, is a cell surface molecule with diverse biologic effects on T cell function. It is an immune checkpoint receptor and as such is the target of various drug development programs by pharmaceutical companies seeking to develop new treatments for cancer and autoimmune disorders. In soluble form it is also being developed as a cancer drug in its own right.

Tolerx, Inc. was a biopharmaceutical company headquartered in Cambridge, Massachusetts. The company was focused on discovering and developing new therapies designed to treat patients by reprogramming the immune system, allowing for long-term remission of immune-related diseases after a short course of therapy. Targeted diseases include type 1 diabetes, rheumatoid arthritis, Inflammatory bowel disease (IBD), cancer, chronic and viral diseases. In 2008, Tolerx was named one of Fierce Biotech’s Fierce 15. In October 2011, Tolerx was shut down due to an unsuccessful Phase III trial in patients recently diagnosed with Type 1 diabetes.

A trifunctional antibody is a monoclonal antibody with binding sites for two different antigens, typically CD3 and a tumor antigen, making it a type of bispecific monoclonal antibody. In addition, its intact Fc-part can bind to an Fc receptor on accessory cells like conventional monospecific antibodies. The net effect is that this type of drug links T cells and monocytes/macrophages, natural killer cells, dendritic cells or other Fc receptor expressing cells to the tumor cells, leading to their destruction.

Two chemically linked fragments antigen-binding form an artificial antibody that binds to two different antigens, making it a type of bispecific antibody. They are fragments antigen-binding of two different monoclonal antibodies and are linked by chemical means like a thioether. Typically, one of the Fabs binds to a tumour antigen and the other to a protein on the surface of an immune cell, for example an Fc receptor on a macrophage. In this way, tumour cells are attached to immune cells, which destroy them.
Short Course Immune Induction Therapy or SCIIT, is a therapeutic strategy employing rapid, specific, short term-modulation of the immune system using a therapeutic agent to induce T-cell non-responsiveness, also known as operational tolerance. As an alternative strategy to immunosuppression and antigen-specific tolerance inducing therapies, the primary goal of SCIIT is to re-establish or induce peripheral immune tolerance in the context of autoimmune disease and transplant rejection through the use of biological agents. In recent years, SCIIT has received increasing attention in clinical and research settings as an alternative to immunosuppressive drugs currently used in the clinic, drugs which put the patients at risk of developing infection, cancer, and cardiovascular disease.
TOL101, is a murine-monoclonal antibody specific for the human αβ T cell receptor. In 2010 it was an Investigational New Drug under development by Tolera Therapeutics, Inc.
Immune checkpoints are regulators of the immune system. These pathways are crucial for self-tolerance, which prevents the immune system from attacking cells indiscriminately.
Infectious tolerance is a term referring to a phenomenon where a tolerance-inducing state is transferred from one cell population to another. It can be induced in many ways; although it is often artificially induced, it is a natural in vivo process. A number of research deal with the development of a strategy utilizing this phenomenon in transplantation immunology. The goal is to achieve long-term tolerance of the transplant through short-term therapy.













