Morphogenesis is the biological process that causes a cell, tissue or organism to develop its shape. It is one of three fundamental aspects of developmental biology along with the control of tissue growth and patterning of cellular differentiation.

A pseudopod or pseudopodium is a temporary arm-like projection of a eukaryotic cell membrane that is emerged in the direction of movement. Filled with cytoplasm, pseudopodia primarily consist of actin filaments and may also contain microtubules and intermediate filaments. Pseudopods are used for motility and ingestion. They are often found in amoebas.

Cytokinesis is the part of the cell division process and part of mitosis during which the cytoplasm of a single eukaryotic cell divides into two daughter cells. Cytoplasmic division begins during or after the late stages of nuclear division in mitosis and meiosis. During cytokinesis the spindle apparatus partitions and transports duplicated chromatids into the cytoplasm of the separating daughter cells. It thereby ensures that chromosome number and complement are maintained from one generation to the next and that, except in special cases, the daughter cells will be functional copies of the parent cell. After the completion of the telophase and cytokinesis, each daughter cell enters the interphase of the cell cycle.

Gastrulation is the stage in the early embryonic development of most animals, during which the blastula, or in mammals the blastocyst, is reorganized into a two-layered or three-layered embryo known as the gastrula. Before gastrulation, the embryo is a continuous epithelial sheet of cells; by the end of gastrulation, the embryo has begun differentiation to establish distinct cell lineages, set up the basic axes of the body, and internalized one or more cell types including the prospective gut.

Microfilaments, also called actin filaments, are protein filaments in the cytoplasm of eukaryotic cells that form part of the cytoskeleton. They are primarily composed of polymers of actin, but are modified by and interact with numerous other proteins in the cell. Microfilaments are usually about 7 nm in diameter and made up of two strands of actin. Microfilament functions include cytokinesis, amoeboid movement, cell motility, changes in cell shape, endocytosis and exocytosis, cell contractility, and mechanical stability. Microfilaments are flexible and relatively strong, resisting buckling by multi-piconewton compressive forces and filament fracture by nanonewton tensile forces. In inducing cell motility, one end of the actin filament elongates while the other end contracts, presumably by myosin II molecular motors. Additionally, they function as part of actomyosin-driven contractile molecular motors, wherein the thin filaments serve as tensile platforms for myosin's ATP-dependent pulling action in muscle contraction and pseudopod advancement. Microfilaments have a tough, flexible framework which helps the cell in movement.

In cell biology, the cleavage furrow is the indentation of the cell's surface that begins the progression of cleavage, by which animal and some algal cells undergo cytokinesis, the final splitting of the membrane, in the process of cell division. The same proteins responsible for muscle contraction, actin and myosin, begin the process of forming the cleavage furrow, creating an actomyosin ring. Other cytoskeletal proteins and actin binding proteins are involved in the procedure.
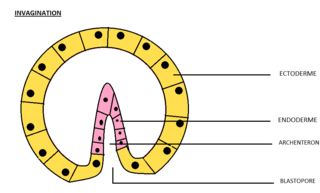
Invagination is the process of a surface folding in on itself to form a cavity, pouch or tube. In developmental biology, invagination is a mechanism that takes place during gastrulation. This mechanism or cell movement happens mostly in the vegetal pole. Invagination consists of the folding of an area of the exterior sheet of cells towards the inside of the blastula. In each organism, the complexity will be different depending on the number of cells. Invagination can be referenced as one of the steps of the establishment of the body plan. The term, originally used in embryology, has been adopted in other disciplines as well.

Neurulation refers to the folding process in vertebrate embryos, which includes the transformation of the neural plate into the neural tube. The embryo at this stage is termed the neurula.
The cell cortex, also known as the actin cortex, cortical cytoskeleton or actomyosin cortex, is a specialized layer of cytoplasmic proteins on the inner face of the cell membrane. It functions as a modulator of membrane behavior and cell surface properties. In most eukaryotic cells lacking a cell wall, the cortex is an actin-rich network consisting of F-actin filaments, myosin motors, and actin-binding proteins. The actomyosin cortex is attached to the cell membrane via membrane-anchoring proteins called ERM proteins that plays a central role in cell shape control. The protein constituents of the cortex undergo rapid turnover, making the cortex both mechanically rigid and highly plastic, two properties essential to its function. In most cases, the cortex is in the range of 100 to 1000 nanometers thick.
An asymmetric cell division produces two daughter cells with different cellular fates. This is in contrast to symmetric cell divisions which give rise to daughter cells of equivalent fates. Notably, stem cells divide asymmetrically to give rise to two distinct daughter cells: one copy of the original stem cell as well as a second daughter programmed to differentiate into a non-stem cell fate.

Epiboly describes one of the five major types of cell movements that occur in the gastrulation stage of embryonic development of some organisms. Epiboly is the spreading and thinning of the ectoderm while the endoderm and mesoderm layers move to the inside of the embryo.
In cell biology, microtubule nucleation is the event that initiates de novo formation of microtubules (MTs). These filaments of the cytoskeleton typically form through polymerization of α- and β-tubulin dimers, the basic building blocks of the microtubule, which initially interact to nucleate a seed from which the filament elongates.

Transforming protein RhoA, also known as Ras homolog family member A (RhoA), is a small GTPase protein in the Rho family of GTPases that in humans is encoded by the RHOA gene. While the effects of RhoA activity are not all well known, it is primarily associated with cytoskeleton regulation, mostly actin stress fibers formation and actomyosin contractility. It acts upon several effectors. Among them, ROCK1 and DIAPH1 are the best described. RhoA, and the other Rho GTPases, are part of a larger family of related proteins known as the Ras superfamily, a family of proteins involved in the regulation and timing of cell division. RhoA is one of the oldest Rho GTPases, with homologues present in the genomes since 1.5 billion years. As a consequence, RhoA is somehow involved in many cellular processes which emerged throughout evolution. RhoA specifically is regarded as a prominent regulatory factor in other functions such as the regulation of cytoskeletal dynamics, transcription, cell cycle progression and cell transformation.

Anillin is a conserved protein implicated in cytoskeletal dynamics during cellularization and cytokinesis. The ANLN gene in humans and the scraps gene in Drosophila encode Anillin. In 1989, anillin was first isolated in embryos of Drosophila melanogaster. It was identified as an F-actin binding protein. Six years later, the anillin gene was cloned from cDNA originating from a Drosophila ovary. Staining with anti-anillin antibody showed the anillin localizes to the nucleus during interphase and to the contractile ring during cytokinesis. These observations agree with further research that found anillin in high concentrations near the cleavage furrow coinciding with RhoA, a key regulator of contractile ring formation.
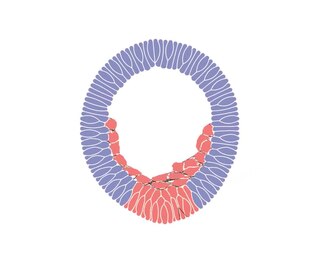
Ingression is one of the many changes in the location or relative position of cells that takes place during the gastrulation stage of embryonic development. It produces an animal's mesenchymal cells at the onset of gastrulation. During the epithelial–mesenchymal transition (EMT), the primary mesenchyme cells (PMCs) detach from the epithelium and become internalized mesenchyme cells that can migrate freely.

Rho-associated protein kinase (ROCK) is a kinase belonging to the AGC family of serine-threonine specific protein kinases. It is involved mainly in regulating the shape and movement of cells by acting on the cytoskeleton.

In molecular biology, an actomyosin ring or contractile ring, is a prominent structure during cytokinesis. It forms perpendicular to the axis of the spindle apparatus towards the end of telophase, in which sister chromatids are identically separated at the opposite sides of the spindle forming nuclei. The actomyosin ring follows an orderly sequence of events: identification of the active division site, formation of the ring, constriction of the ring, and disassembly of the ring. It is composed of actin and myosin II bundles, thus the term actomyosin. The actomyosin ring operates in contractile motion, although the mechanism on how or what triggers the constriction is still an evolving topic. Other cytoskeletal proteins are also involved in maintaining the stability of the ring and driving its constriction. Apart from cytokinesis, in which the ring constricts as the cells divide, actomyosin ring constriction has also been found to activate during wound closure. During this process, actin filaments are degraded, preserving the thickness of the ring. After cytokinesis is complete, one of the two daughter cells inherits a remnant known as the midbody ring.

The dorsal lip of the blastopore is a structure that forms during early embryonic development and is important for its role in organizing the germ layers. The dorsal lip is formed during early gastrulation as folding of tissue along the involuting marginal zone of the blastocoel forms an opening known as the blastopore. It is particularly important for its role in neural induction through the default model, where signaling from the dorsal lip protects a region of the epiblast from becoming epidermis, thus allowing it to develop to its default neural tissue.
Barry James Thompson is an Australian and British developmental biologist and cancer biologist. Thompson is known for identifying genes, proteins and mechanisms involved in epithelial polarity, morphogenesis and cell signaling via the Wnt and Hippo signaling pathways, which have key roles in human cancer.
Edwin W. Taylor is an adjunct professor of cell and developmental biology at Northwestern University. He was elected to the National Academy of Sciences in 2001. Taylor received a BA in physics and chemistry from the University of Toronto in 1952; an MSc in physical chemistry from McMaster University in 1955, and a PhD in biophysics from the University of Chicago in 1957. In 2001 Taylor was elected to the National Academy of Sciences in Cellular and Developmental Biology and Biochemistry.


















