Ontogeny is the origination and development of an organism, usually from the time of fertilization of the egg to adult. The term can also be used to refer to the study of the entirety of an organism's lifespan.
The development of the nervous system, or neural development (neurodevelopment), refers to the processes that generate, shape, and reshape the nervous system of animals, from the earliest stages of embryonic development to adulthood. The field of neural development draws on both neuroscience and developmental biology to describe and provide insight into the cellular and molecular mechanisms by which complex nervous systems develop, from nematodes and fruit flies to mammals.

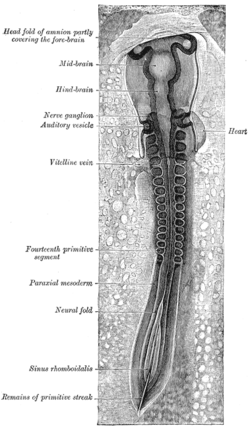
In the developing chordate, the neural tube is the embryonic precursor to the central nervous system, which is made up of the brain and spinal cord. The neural groove gradually deepens as the neural fold become elevated, and ultimately the folds meet and coalesce in the middle line and convert the groove into the closed neural tube. In humans, neural tube closure usually occurs by the fourth week of pregnancy.

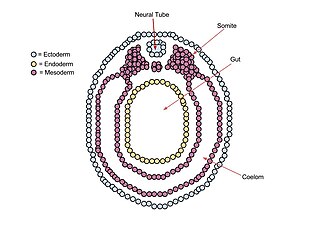
The ectoderm is one of the three primary germ layers formed in early embryonic development. It is the outermost layer, and is superficial to the mesoderm and endoderm. It emerges and originates from the outer layer of germ cells. The word ectoderm comes from the Greek ektos meaning "outside", and derma meaning "skin".

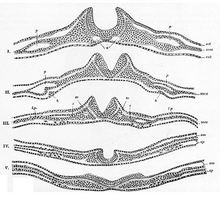
Neurulation refers to the folding process in vertebrate embryos, which includes the transformation of the neural plate into the neural tube. The embryo at this stage is termed the neurula.
A germ layer is a primary layer of cells that forms during embryonic development. The three germ layers in vertebrates are particularly pronounced; however, all eumetazoans produce two or three primary germ layers. Some animals, like cnidarians, produce two germ layers making them diploblastic. Other animals such as bilaterians produce a third layer between these two layers, making them triploblastic. Germ layers eventually give rise to all of an animal's tissues and organs through the process of organogenesis.
Organogenesis is the phase of embryonic development that starts at the end of gastrulation and continues until birth. During organogenesis, the three germ layers formed from gastrulation form the internal organs of the organism.

The neural plate is a key developmental structure that serves as the basis for the nervous system. Cranial to the primitive node of the embryonic primitive streak, ectodermal tissue thickens and flattens to become the neural plate. The region anterior to the primitive node can be generally referred to as the neural plate. Cells take on a columnar appearance in the process as they continue to lengthen and narrow. The ends of the neural plate, known as the neural folds, push the ends of the plate up and together, folding into the neural tube, a structure critical to brain and spinal cord development. This process as a whole is termed primary neurulation.
A neurula is a vertebrate embryo at the early stage of development in which neurulation occurs. The neurula stage is preceded by the gastrula stage; consequentially, neurulation is preceded by gastrulation. Neurulation marks the beginning of the process of organogenesis.

Neural crest cells are a temporary group of cells that arise from the embryonic ectoderm germ layer, and in turn give rise to a diverse cell lineage—including melanocytes, craniofacial cartilage and bone, smooth muscle, peripheral and enteric neurons and glia.
The primitive node is the organizer for gastrulation in most amniote embryos. In birds it is known as Hensen's node, and in amphibians it is known as the Spemann-Mangold organizer. It is induced by the Nieuwkoop center in amphibians, or by the posterior marginal zone in amniotes including birds.

Paraxial mesoderm, also known as presomitic or somitic mesoderm, is the area of mesoderm in the neurulating embryo that flanks and forms simultaneously with the neural tube. The cells of this region give rise to somites, blocks of tissue running along both sides of the neural tube, which form muscle and the tissues of the back, including connective tissue and the dermis.

The neural groove is a shallow median groove of the neural plate between the neural folds of an embryo. The neural plate is a thick sheet of ectoderm surrounded on either side by the neural folds, two longitudinal ridges in front of the primitive streak of the developing embryo.

Neuroectoderm consists of cells derived from the ectoderm. Formation of the neuroectoderm is the first step in the development of the nervous system. The neuroectoderm receives bone morphogenetic protein-inhibiting signals from proteins such as noggin, which leads to the development of the nervous system from this tissue. Histologically, these cells are classified as pseudostratified columnar cells.

Mesenchyme is a type of loosely organized animal embryonic connective tissue of undifferentiated cells that give rise to most tissues, such as skin, blood or bone. The interactions between mesenchyme and epithelium help to form nearly every organ in the developing embryo.
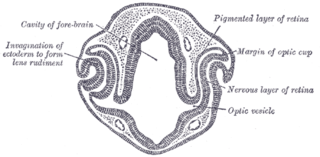
Eye formation in the human embryo begins at approximately three weeks into embryonic development and continues through the tenth week. Cells from both the mesodermal and the ectodermal tissues contribute to the formation of the eye. Specifically, the eye is derived from the neuroepithelium, surface ectoderm, and the extracellular mesenchyme which consists of both the neural crest and mesoderm.

The development of fishes is unique in some specific aspects compared to the development of other animals.

Human embryonic development or human embryogenesis is the development and formation of the human embryo. It is characterised by the processes of cell division and cellular differentiation of the embryo that occurs during the early stages of development. In biological terms, the development of the human body entails growth from a one-celled zygote to an adult human being. Fertilization occurs when the sperm cell successfully enters and fuses with an egg cell (ovum). The genetic material of the sperm and egg then combine to form the single cell zygote and the germinal stage of development commences. Embryonic development in the human, covers the first eight weeks of development; at the beginning of the ninth week the embryo is termed a fetus. The eight weeks have 23 stages.
The rostral neuropore or anterior neuropore is a region corresponding to the opening of the embryonic neural tube in the anterior portion of the developing prosencephalon. The central nervous system develops from the neural tube, which initially starts as a plate of cells in the ectoderm and this is called the neural plate, the neural plate then undergoes folding and starts closing from the center of the developing fetus, this leads to two open ends, one situated cranially/rostrally and the other caudally. Bending of the neural plate begins on day 22, and the cranial neuropore closes on day 24. giving rise to the lamina terminalis of the brain.
Neural crest cells are multipotent cells required for the development of cells, tissues and organ systems. A subpopulation of neural crest cells are the cardiac neural crest complex. This complex refers to the cells found amongst the midotic placode and somite 3 destined to undergo epithelial-mesenchymal transformation and migration to the heart via pharyngeal arches 3, 4 and 6.