Related Research Articles

The cerebral cortex, also known as the cerebral mantle, is the outer layer of neural tissue of the cerebrum of the brain in humans and other mammals. It is the largest site of neural integration in the central nervous system, and plays a key role in attention, perception, awareness, thought, memory, language, and consciousness. The cerebral cortex is the part of the brain responsible for cognition.
The development of the nervous system, or neural development (neurodevelopment), refers to the processes that generate, shape, and reshape the nervous system of animals, from the earliest stages of embryonic development to adulthood. The field of neural development draws on both neuroscience and developmental biology to describe and provide insight into the cellular and molecular mechanisms by which complex nervous systems develop, from nematodes and fruit flies to mammals.
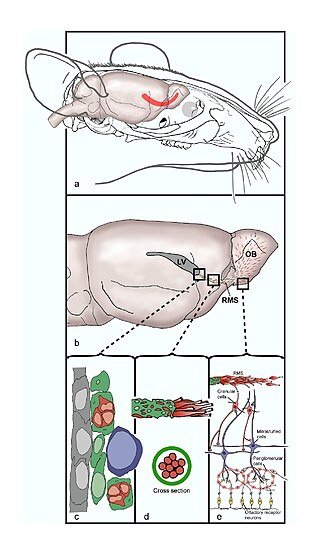
The rostral migratory stream (RMS) is a specialized migratory route found in the brain of some animals along which neuronal precursors that originated in the subventricular zone (SVZ) of the brain migrate to reach the main olfactory bulb (OB). The importance of the RMS lies in its ability to refine and even change an animal's sensitivity to smells, which explains its importance and larger size in the rodent brain as compared to the human brain, as our olfactory sense is not as developed. This pathway has been studied in the rodent, rabbit, and both the squirrel monkey and rhesus monkey. When the neurons reach the OB they differentiate into GABAergic interneurons as they are integrated into either the granule cell layer or periglomerular layer.
Neuroepithelial cells, or neuroectodermal cells, form the wall of the closed neural tube in early embryonic development. The neuroepithelial cells span the thickness of the tube's wall, connecting with the pial surface and with the ventricular or lumenal surface. They are joined at the lumen of the tube by junctional complexes, where they form a pseudostratified layer of epithelium called neuroepithelium.
Neural stem cells (NSCs) are self-renewing, multipotent cells that firstly generate the radial glial progenitor cells that generate the neurons and glia of the nervous system of all animals during embryonic development. Some neural progenitor stem cells persist in highly restricted regions in the adult vertebrate brain and continue to produce neurons throughout life. Differences in the size of the central nervous system are among the most important distinctions between the species and thus mutations in the genes that regulate the size of the neural stem cell compartment are among the most important drivers of vertebrate evolution.

Radial glial cells, or radial glial progenitor cells (RGPs), are bipolar-shaped progenitor cells that are responsible for producing all of the neurons in the cerebral cortex. RGPs also produce certain lineages of glia, including astrocytes and oligodendrocytes. Their cell bodies (somata) reside in the embryonic ventricular zone, which lies next to the developing ventricular system.

The subventricular zone (SVZ) is a region situated on the outside wall of each lateral ventricle of the vertebrate brain. It is present in both the embryonic and adult brain. In embryonic life, the SVZ refers to a secondary proliferative zone containing neural progenitor cells, which divide to produce neurons in the process of neurogenesis. The primary neural stem cells of the brain and spinal cord, termed radial glial cells, instead reside in the ventricular zone (VZ).
An asymmetric cell division produces two daughter cells with different cellular fates. This is in contrast to symmetric cell divisions which give rise to daughter cells of equivalent fates. Notably, stem cells divide asymmetrically to give rise to two distinct daughter cells: one copy of the original stem cell as well as a second daughter programmed to differentiate into a non-stem cell fate.

The subgranular zone (SGZ) is a brain region in the hippocampus where adult neurogenesis occurs. The other major site of adult neurogenesis is the subventricular zone (SVZ) in the brain.

Protein numb homolog is a protein that in humans is encoded by the NUMB gene. The protein encoded by this gene plays a role in the determination of cell fates during development. The encoded protein, whose degradation is induced in a proteasome-dependent manner by MDM2, is a membrane-bound protein that has been shown to associate with EPS15, LNX1, and NOTCH1. Four transcript variants encoding different isoforms have been found for this gene.

Ganglion mother cells (GMCs) are cells involved in neurogenesis, in non-mammals, that divide only once to give rise to two neurons, or one neuron and one glial cell or two glial cells, and are present only in the central nervous system. They are also responsible for transcription factor expression. While each ganglion mother cell necessarily gives rise to two neurons, a neuroblast can asymmetrically divide multiple times. GMCs are the progeny of type I neuroblasts. Neuroblasts asymmetrically divide during embryogenesis to create GMCs. GMCs are only present in certain species and only during the embryonic and larval stages of life. Recent research has shown that there is an intermediate stage between a GMC and two neurons. The GMC forms two ganglion cells which then develop into neurons or glial cells. Embryonic neurogenesis has been extensively studied in Drosophila melanogaster embryos and larvae.

Eomesodermin also known as T-box brain protein 2 (Tbr2) is a protein that in humans is encoded by the EOMES gene.
Endogenous regeneration in the brain is the ability of cells to engage in the repair and regeneration process. While the brain has a limited capacity for regeneration, endogenous neural stem cells, as well as numerous pro-regenerative molecules, can participate in replacing and repairing damaged or diseased neurons and glial cells. Another benefit that can be achieved by using endogenous regeneration could be avoiding an immune response from the host.
The development of the cerebral cortex, known as corticogenesis is the process during which the cerebral cortex of the brain is formed as part of the development of the nervous system of mammals including its development in humans. The cortex is the outer layer of the brain and is composed of up to six layers. Neurons formed in the ventricular zone migrate to their final locations in one of the six layers of the cortex. The process occurs from embryonic day 10 to 17 in mice and between gestational weeks seven to 18 in humans.
Proneural genes encode transcription factors of the basic helix-loop-helix (bHLH) class which are responsible for the development of neuroectodermal progenitor cells. Proneural genes have multiple functions in neural development. They integrate positional information and contribute to the specification of progenitor-cell identity. From the same ectodermal cell types, neural or epidermal cells can develop based on interactions between proneural and neurogenic genes. Neurogenic genes are so called because loss of function mutants show an increase number of developed neural precursors. On the other hand, proneural genes mutants fail to develop neural precursor cells.

A neuronal lineage marker is an endogenous tag that is expressed in different cells along neurogenesis and differentiated cells such as neurons. It allows detection and identification of cells by using different techniques. A neuronal lineage marker can be either DNA, mRNA or RNA expressed in a cell of interest. It can also be a protein tag, as a partial protein, a protein or an epitope that discriminates between different cell types or different states of a common cell. An ideal marker is specific to a given cell type in normal conditions and/or during injury. Cell markers are very valuable tools for examining the function of cells in normal conditions as well as during disease. The discovery of various proteins specific to certain cells led to the production of cell-type-specific antibodies that have been used to identify cells.
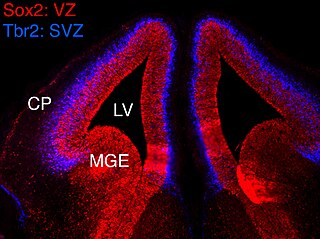
In vertebrates, the ventricular zone (VZ) is a transient embryonic layer of tissue containing neural stem cells, principally radial glial cells, of the central nervous system (CNS). The VZ is so named because it lines the ventricular system, which contains cerebrospinal fluid (CSF). The embryonic ventricular system contains growth factors and other nutrients needed for the proper function of neural stem cells. Neurogenesis, or the generation of neurons, occurs in the VZ during embryonic and fetal development as a function of the Notch pathway, and the newborn neurons must migrate substantial distances to their final destination in the developing brain or spinal cord where they will establish neural circuits. A secondary proliferative zone, the subventricular zone (SVZ), lies adjacent to the VZ. In the embryonic cerebral cortex, the SVZ contains intermediate neuronal progenitors that continue to divide into post-mitotic neurons. Through the process of neurogenesis, the parent neural stem cell pool is depleted and the VZ disappears. The balance between the rates of stem cell proliferation and neurogenesis changes during development, and species from mouse to human show large differences in the number of cell cycles, cell cycle length, and other parameters, which is thought to give rise to the large diversity in brain size and structure.
Neurogenesis is the process by which nervous system cells, the neurons, are produced by neural stem cells (NSCs). This occurs in all species of animals except the porifera (sponges) and placozoans. Types of NSCs include neuroepithelial cells (NECs), radial glial cells (RGCs), basal progenitors (BPs), intermediate neuronal precursors (INPs), subventricular zone astrocytes, and subgranular zone radial astrocytes, among others.
Intermediate progenitor cells (IPCs) are a type of progenitor cell in the developing cerebral cortex. They are multipolar cells produced by radial glial cells who have undergone asymmetric division. IPCs can produce neuron cells via neurogenesis and are responsible for ensuring the proper quantity of cortical neurons are produced. In mammals, neural stem cells are the primary progenitors during embryogenesis whereas intermediate progenitor cells are the secondary progenitors.
Arnold Richard Kriegstein is a neurologist and neuroscientist at the University of California, San Francisco, where he served as director of the UCSF Eli and Edythe Broad Center of Regeneration Medicine and Stem Cell Research from 2004 to 2021. His main research interests include neural stem cell and brain development. He is a member of the National Academy of Medicine.
References
- 1 2 Sadler, T. (2010). Langman's medical embryology (11th ed.). Philadelphia: Lippincott William & Wilkins. pp. 296–297. ISBN 978-07817-9069-7.
- ↑ Williams, S. Mark (2001). "The Initial Formation of the Nervous System: Gastrulation and Neurulation". Neuroscience. 2nd edition. Retrieved 5 January 2019.
- 1 2 Purves, Dale (2012). Neuroscience (5th ed.). Sinauer Associates. p. 490. ISBN 9780878936953.
- ↑ "wberesford.hsc.wvu.edu" . Retrieved 2010-04-08.
- 1 2 3 Johnson, CA; Wright, CE; Ghashghaei, HT (December 2017). "Regulation of cytokinesis during corticogenesis: focus on the midbody". FEBS Letters. 591 (24): 4009–4026. doi: 10.1002/1873-3468.12676 . PMID 28493553.
- ↑ Gilbert, Scott (2006). Developmental biology (8th ed.). Sinauer Associates Publishers. pp. 386–387. ISBN 9780878932504.
- ↑ Purves, D; et al. (2007). Neuroscience (4th ed.). New York: W. H. Freeman. ISBN 978-0-87893-697-7.[ page needed ]
- ↑ Tortora, G; Derrickson, B (2011). Principles of anatomy & physiology (13th. ed.). Wiley. p. 571. ISBN 9780470646083.
- ↑ Liu, F; You, Y; Li, X; Ma, T; Nie, Y; Wei, B; Li, T; Lin, H; Yang, Z (April 2009). "Brain Injury Does Not Alter the Intrinsic Differentiation Potential of Adult Neuroblasts". The Journal of Neuroscience. 29 (16): 5075–5087. doi: 10.1523/JNEUROSCI.0201-09.2009 . PMC 6665479 . PMID 19386903.
- ↑ Gallaud, E; Pham, T; Cabernard, C (2017). "Drosophila melanogaster Neuroblasts: A Model for Asymmetric Stem Cell Divisions". Asymmetric Cell Division in Development, Differentiation and Cancer. Results and Problems in Cell Differentiation. Vol. 61. pp. 183–210. doi:10.1007/978-3-319-53150-2_8. ISBN 978-3-319-53149-6. PMID 28409305.
- 1 2 3 Doe, Chris Q. (2017-10-06). "Temporal Patterning in the Drosophila CNS". Annual Review of Cell and Developmental Biology. 33 (1): 219–240. doi: 10.1146/annurev-cellbio-111315-125210 . ISSN 1081-0706. PMID 28992439.
- ↑ Courgeon, Maximilien; Desplan, Claude (2019-06-01). "Coordination of neural patterning in the Drosophila visual system". Current Opinion in Neurobiology. Neuronal Identity. 56: 153–159. doi:10.1016/j.conb.2019.01.024. ISSN 0959-4388. PMC 6551251 . PMID 30849690.
- ↑ Sen, Sonia Q; Chanchani, Sachin; Southall, Tony D; Doe, Chris Q (2019-01-29). Mandel, Gail; Struhl, Kevin; Desplan, Claude; Eisen, Michael B (eds.). "Neuroblast-specific open chromatin allows the temporal transcription factor, Hunchback, to bind neuroblast-specific loci". eLife. 8: e44036. doi: 10.7554/eLife.44036 . ISSN 2050-084X. PMC 6377230 . PMID 30694180.
- ↑ Kohwi, M; Hiebert, LS; Doe, CQ (May 2011). "The pipsqueak-domain proteins Distal antenna and Distal antenna-related restrict Hunchback neuroblast expression and early-born neuronal identity". Development. 138 (9): 1727–35. doi:10.1242/dev.061499. PMC 3074449 . PMID 21429984.