Related Research Articles
The development of the nervous system, or neural development, or neurodevelopment, refers to the processes that generate, shape, and reshape the nervous system of animals, from the earliest stages of embryonic development to adulthood. The field of neural development draws on both neuroscience and developmental biology to describe and provide insight into the cellular and molecular mechanisms by which complex nervous systems develop, from nematodes and fruit flies to mammals.

Adult neurogenesis is the process in which neurons are generated from neural stem cells in the adult. This process differs from prenatal neurogenesis.
In vertebrates, a neuroblast or primitive nerve cell is a postmitotic cell that does not divide further, and which will develop into a neuron after a migration phase. In invertebrates such as Drosophila, neuroblasts are neural progenitor cells which divide asymmetrically to produce a neuroblast, and a daughter cell of varying potency depending on the type of neuroblast. Vertebrate neuroblasts differentiate from radial glial cells and are committed to becoming neurons. Neural stem cells, which only divide symmetrically to produce more neural stem cells, transition gradually into radial glial cells. Radial glial cells, also called radial glial progenitor cells, divide asymmetrically to produce a neuroblast and another radial glial cell that will re-enter the cell cycle.

The rostral migratory stream (RMS) is a specialized migratory route found in the brain of some animals along which neuronal precursors that originated in the subventricular zone (SVZ) of the brain migrate to reach the main olfactory bulb (OB). The importance of the RMS lies in its ability to refine and even change an animal's sensitivity to smells, which explains its importance and larger size in the rodent brain as compared to the human brain, as our olfactory sense is not as developed. This pathway has been studied in the rodent, rabbit, and both the squirrel monkey and rhesus monkey. When the neurons reach the OB they differentiate into GABAergic interneurons as they are integrated into either the granule cell layer or periglomerular layer.
Neuroepithelial cells, or neuroectodermal cells, form the wall of the closed neural tube in early embryonic development. The neuroepithelial cells span the thickness of the tube's wall, connecting with the pial surface and with the ventricular or lumenal surface. They are joined at the lumen of the tube by junctional complexes, where they form a pseudostratified layer of epithelium called neuroepithelium.
Neural stem cells (NSCs) are self-renewing, multipotent cells that firstly generate the radial glial progenitor cells that generate the neurons and glia of the nervous system of all animals during embryonic development. Some neural progenitor stem cells persist in highly restricted regions in the adult vertebrate brain and continue to produce neurons throughout life. Differences in the size of the central nervous system are among the most important distinctions between the species and thus mutations in the genes that regulate the size of the neural stem cell compartment are among the most important drivers of vertebrate evolution.

Radial glial cells, or radial glial progenitor cells (RGPs), are bipolar-shaped progenitor cells that are responsible for producing all of the neurons in the cerebral cortex. RGPs also produce certain lineages of glia, including astrocytes and oligodendrocytes. Their cell bodies (somata) reside in the embryonic ventricular zone, which lies next to the developing ventricular system.

The subventricular zone (SVZ) is a region situated on the outside wall of each lateral ventricle of the vertebrate brain. It is present in both the embryonic and adult brain. In embryonic life, the SVZ refers to a secondary proliferative zone containing neural progenitor cells, which divide to produce neurons in the process of neurogenesis. The primary neural stem cells of the brain and spinal cord, termed radial glial cells, instead reside in the ventricular zone (VZ).
Michael S. Kaplan is an American biology researcher, medical professor, and clinical physician. A pioneer of neurogenesis research, his work refuted the classic idea that no new nerve cells are born in the adult mammalian brain. His research using light and electron microscopy suggested that neurogenesis occurs in the brain of adult mammals, but his findings were rejected by the scientific community at the time in a field that continues to be contentious. Doctor Kaplan has recently begun a YouTube channel which offers patient interviews and insights to brain plasticity; kaplan brain health, YouTube.

The subgranular zone (SGZ) is a brain region in the hippocampus where adult neurogenesis occurs. The other major site of adult neurogenesis is the subventricular zone (SVZ) in the brain.
Radiation-induced cognitive decline describes the possible correlation between radiation therapy and cognitive impairment. Radiation therapy is used mainly in the treatment of cancer. Radiation therapy can be used to cure care or shrink tumors that are interfering with quality of life. Sometimes radiation therapy is used alone; other times it is used in conjunction with chemotherapy and surgery. For people with brain tumors, radiation can be an effective treatment because chemotherapy is often less effective due to the blood–brain barrier. Unfortunately for some patients, as time passes, people who received radiation therapy may begin experiencing deficits in their learning, memory, and spatial information processing abilities. The learning, memory, and spatial information processing abilities are dependent on proper hippocampus functionality. Therefore, any hippocampus dysfunction will result in deficits in learning, memory, and spatial information processing ability.
Gyrification is the process of forming the characteristic folds of the cerebral cortex.
Gliogenesis is the generation of non-neuronal glia populations derived from multipotent neural stem cells.
Endogenous regeneration in the brain is the ability of cells to engage in the repair and regeneration process. While the brain has a limited capacity for regeneration, endogenous neural stem cells, as well as numerous pro-regenerative molecules, can participate in replacing and repairing damaged or diseased neurons and glial cells. Another benefit that can be achieved by using endogenous regeneration could be avoiding an immune response from the host.

Corticogenesis is the process in which the cerebral cortex of the brain is formed during the development of the nervous system. The cortex is the outer layer of the brain and is composed of up to six layers. Neurons formed in the ventricular zone migrate to their final locations in one of the six layers of the cortex. The process occurs from embryonic day 10 to 17 in mice and between gestational weeks seven to 18 in humans.
Epigenetics is the study of heritable changes in gene expression which do not result from modifications to the sequence of DNA. Neurogenesis is the mechanism for neuron proliferation and differentiation. It entails many different complex processes which are all time and order dependent. Processes such as neuron proliferation, fate specification, differentiation, maturation, and functional integration of newborn cells into existing neuronal networks are all interconnected. In the past decade many epigenetic regulatory mechanisms have been shown to play a large role in the timing and determination of neural stem cell lineages.

A neuronal lineage marker is an endogenous tag that is expressed in different cells along neurogenesis and differentiated cells such as neurons. It allows detection and identification of cells by using different techniques. A neuronal lineage marker can be either DNA, mRNA or RNA expressed in a cell of interest. It can also be a protein tag, as a partial protein, a protein or an epitope that discriminates between different cell types or different states of a common cell. An ideal marker is specific to a given cell type in normal conditions and/or during injury. Cell markers are very valuable tools for examining the function of cells in normal conditions as well as during disease. The discovery of various proteins specific to certain cells led to the production of cell-type-specific antibodies that have been used to identify cells.
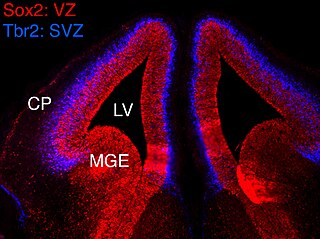
In vertebrates, the ventricular zone (VZ) is a transient embryonic layer of tissue containing neural stem cells, principally radial glial cells, of the central nervous system (CNS). The VZ is so named because it lines the ventricular system, which contains cerebrospinal fluid (CSF). The embryonic ventricular system contains growth factors and other nutrients needed for the proper function of neural stem cells. Neurogenesis, or the generation of neurons, occurs in the VZ during embryonic and fetal development as a function of the Notch pathway, and the newborn neurons must migrate substantial distances to their final destination in the developing brain or spinal cord where they will establish neural circuits. A secondary proliferative zone, the subventricular zone (SVZ), lies adjacent to the VZ. In the embryonic cerebral cortex, the SVZ contains intermediate neuronal progenitors that continue to divide into post-mitotic neurons. Through the process of neurogenesis, the parent neural stem cell pool is depleted and the VZ disappears. The balance between the rates of stem cell proliferation and neurogenesis changes during development, and species from mouse to human show large differences in the number of cell cycles, cell cycle length, and other parameters, which is thought to give rise to the large diversity in brain size and structure.
Neurogenesis is the process by which nervous system cells, the neurons, are produced by neural stem cells (NSCs). It occurs in all species of animals except the porifera (sponges) and placozoans. Types of NSCs include neuroepithelial cells (NECs), radial glial cells (RGCs), basal progenitors (BPs), intermediate neuronal precursors (INPs), subventricular zone astrocytes, and subgranular zone radial astrocytes, among others.
Intermediate progenitor cells (IPCs) are a type of progenitor cell in the developing cerebral cortex. They are multipolar cells produced by radial glial cells who have undergone asymmetric division. IPCs can produce neuron cells via neurogenesis and are responsible for ensuring the proper quantity of cortical neurons are produced. In mammals, neural stem cells are the primary progenitors during embryogenesis whereas intermediate progenitor cells are the secondary progenitors.
References
- ↑ Joseph, Altman; Das, GD (1965). "Autoradiographic and Histological Evidence of Postnatal Hippocampal Neurogenesis in Rats". Journal of Comparative Neurology. 124 (3): 319–35. doi:10.1002/cne.901240303. PMID 5861717. S2CID 14121873.
- ↑ Goldman, Steven; F Nottebohm (1983). "Neuronal production, migration, and differentiation in a vocal control nucleus of the adult female canary brain". Proceedings of the National Academy of Sciences. 80 (8): 2390–2394. doi: 10.1073/pnas.80.8.2390 . PMC 393826 . PMID 6572982.
- 1 2 3 Goldman, Steven (1999). "Adult neurogenesis: From canaries to the clinic". Journal of Neurobiology. 36 (2): 267–286. doi:10.1002/(SICI)1097-4695(199808)36:2<267::AID-NEU12>3.0.CO;2-B. PMID 9712309.
- ↑ Scheffler, Bjorn; Noah Walton; Dean Lin; Katrin Goetz; Grigori Enikolopov; Steve Roper; Dennis Steindler (2005). "Phenotypic and functional characterization of adult brain neuropoiesis". PNAS. 102 (26): 9353–9358. doi: 10.1073/pnas.0503965102 . PMC 1150897 . PMID 15961540.
- 1 2 3 Scheffler, Bjorn; Meyer Horn; Ingmar Blumcke; Eric Laywell; Debra Coomes; Valery Kukekov; Dennis Steindler (1999). "Marrow-mindedness: a perspective on neuropoiesis". Trends in Neurosciences. 22 (8): 348–357. doi:10.1016/S0166-2236(99)01416-2. PMID 10407420. S2CID 6979065.
- 1 2 3 AV, Terskith; M Easterday; L Li; L Hood; H Kornblum; D Geschwind; I Weissman (2002). "From hematopoiesis to neuropoiesis: evidence of overlapping genetic programs". Journal of Neurochemistry. 81 (14): 7934–7939. doi:10.1046/j.1471-4159.81.s1.105.x. PMC 35446 . PMID 11438738.
- ↑ Theodorou, E; G Dalembert; C Heffelfinger; E White; S Weissman; L Corcoran; M Snyder (2009). "Revert field A high throughput embryonic stem cell screen identifies Oct-2 as a bifunctional regulator of neuronal differentiation". Genes & Development. 23 (5): 575–588. doi:10.1101/gad.1772509. PMC 2658525 . PMID 19270158.
- 1 2 Wen Shu; Li Hong; Liu Jia (2009). "Epigenetic background of neuronal fate determination". Progress in Neurobiology. 87 (2): 98–117. doi:10.1016/j.pneurobio.2008.10.002. PMID 19007844. S2CID 207406250.
- 1 2 Kim, W Y; J Shen (2008). "Presenilins are required for maintenance of neural stem cells in the developing brain". Molecular Neurodegeneration. 3: 2. doi:10.1186/1750-1326-3-2. PMC 2235867 . PMID 18182109.
- ↑ Park, I H; R Zhao; A West; A Yabuuchi; H Huo; Ince T; P Lerou; M Lensch; G Daley (2008). "Reprogramming of human somatic cells to pluripotency with defined factors". Nature. 451 (7175): 141–6. doi:10.1038/nature06534. PMID 18157115. S2CID 4380451.
- ↑ Porayette, P; M gallego; M Kaltcheva; R Bowen; S Meethal; C Atwood (2009). "Differential Processing of Amyloid-beta Precursor Protein Directs Human Embryonic Stem Cell Proliferation and Differentiation into Neuronal Precursor Cells". Journal of Biological Chemistry. 284 (35): 23806–23817. doi: 10.1074/jbc.M109.026328 . PMC 2749153 . PMID 19542221.