 | |
| Identifiers | |
|---|---|
3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
PubChem CID | |
| |
| |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Aplyzanzine A is a bio-active isolate of marine sponge. [2]
 | |
| Identifiers | |
|---|---|
3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
PubChem CID | |
| |
| |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Aplyzanzine A is a bio-active isolate of marine sponge. [2]

A spongivore is an animal anatomically and physiologically adapted to eating animals of the phylum Porifera, commonly called sea sponges, for the main component of its diet. As a result of their diet, spongivore animals like the hawksbill turtle have developed sharp, narrow bird-like beak that allows them to reach within crevices on the reef to obtain sponges.

5-Bromo-DMT (5-bromo-N,N-dimethyltryptamine) is a psychedelic brominated indole alkaloid found in the sponges Smenospongia aurea and Smenospongia echina, as well as in Verongula rigida alongside 5,6-Dibromo-DMT and seven other alkaloids. It is the 5-bromo derivative of DMT, a psychedelic found in many plants and animals.
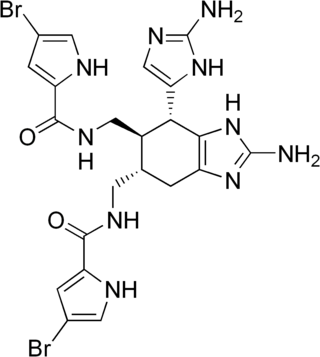
Ageliferin is a chemical compound produced by some sponges. It was first isolated from Caribbean and then Okinawan marine sponges in the genus Agelas. It often co-exists with the related compound sceptrin and other similar compounds. It has antibacterial properties and can cause biofilms to dissolve.

Ircinia strobilina is a species of sponge in the family Irciniidae. It is grey or shiny black in colour, with spiny structures (conules) dotting the surface. The spiny structures are interconnected by ridges, though not arranged in an orderly lattice. This species is globular and massive in shape, but usually no more than 0.3 metres (1 ft) across. I. strobilina is lobed and spherical and has a tough consistency. The large excurrent pores are located in depressions at the top of the sponge. Many smaller incurrent pores are scattered across the surface, more densely at the sides.

Chemical defense is a strategy employed by many organisms to avoid consumption by producing toxic or repellent metabolites or chemical warnings which incite defensive behavioral changes. The production of defensive chemicals occurs in plants, fungi, and bacteria, as well as invertebrate and vertebrate animals. The class of chemicals produced by organisms that are considered defensive may be considered in a strict sense to only apply to those aiding an organism in escaping herbivory or predation. However, the distinction between types of chemical interaction is subjective and defensive chemicals may also be considered to protect against reduced fitness by pests, parasites, and competitors. Repellent rather than toxic metabolites are allomones, a sub category signaling metabolites known as semiochemicals. Many chemicals used for defensive purposes are secondary metabolites derived from primary metabolites which serve a physiological purpose in the organism. Secondary metabolites produced by plants are consumed and sequestered by a variety of arthropods and, in turn, toxins found in some amphibians, snakes, and even birds can be traced back to arthropod prey. There are a variety of special cases for considering mammalian antipredatory adaptations as chemical defenses as well.

Amphimedon compressa, the erect rope sponge, red tree sponge, red tubular sponge, or red sponge is a demosponge found in southern Florida, the Caribbean Sea, and the Bahamas. It can be deep red, orange, brown, or black.

Ianthella basta is a species of fan-shaped sea sponge in the class Demospongiae. It is also known as the elephant ear sponge, paper sponge, or scroll sponge.
Sea sponge aquaculture is the process of farming sea sponges under controlled conditions. It has been conducted in the world's oceans for centuries using a number of aquaculture techniques. There are many factors such as light, salinity, pH, dissolved oxygen and the accumulation of waste products that influence the growth rate of sponges. The benefits of sea sponge aquaculture are realised as a result of its ease of establishment, minimum infrastructure requirements and the potential to be used as a source of income for populations living in developing countries. Sea sponges are produced on a commercial scale to be used as bath sponges or to extract biologically active compounds which are found in certain sponge species. Techniques such as the rope and mesh bag method are used to culture sponges independently or within an integrated multi-trophic aquaculture system setting. One of the only true sustainable sea sponges cultivated in the world occur in the region of Micronesia, with a number of growing and production methods used to ensure and maintain the continued sustainability of these farmed species.
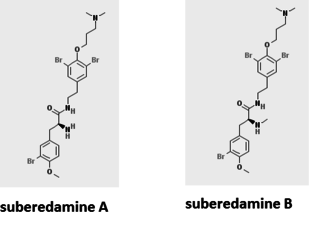
Suberedamines are bio-active isolates of Suberea, a marine sponge. The compounds are brominated tyrosine dimer derivatives.

Aplysamine-2 is a bio-active isolate of marine sponge.

Stevensine is a bromopyrrole alkaloid originally isolated from an unidentified Micronesian marine sponge, as well as a New Caledonian sponge, Pseudaxinyssa cantharella and Axinella corrugata. Total synthesis of stevensine has been achieved by Ying-zi Xu et al., and investigations into the biosynthetic origin has been explored by Paul Andrade et al. Understanding methods to synthesize stevensine and other similar compounds is an important step to accomplish, as marine sponges contain numerous biologically active metabolites that have been shown to function as anything from antitumor to antibacterial agents when tested for medicinal applications. Reasons for why marine sponges contain so many bio-active chemicals has been attributed to their sessile nature, and the need to produce chemical defenses to ensure survival. However, since many of these compounds naturally occur in small amounts, harvesting the sponges has in the past led to near-extinction of some species.

Oroidin is a bromopyrrole alkaloid, originally isolated from marine sponges in the genus Agelas. Its complex structure leads to wide biological activities, which makes Oroidin a potential drug candidate for various diseases. It also serves as chemical defense in marine sponges.

Lacking an immune system, protective shell, or mobility, sponges have developed an ability to synthesize a variety of unusual compounds for survival. C-nucleosides isolated from Caribbean Cryptotethya crypta, were the basis for the synthesis of zidovudine (AZT), aciclovir (Cyclovir), cytarabine (Depocyt), and cytarabine derivative gemcitabine (Gemzar).

Plakoridine A is an alkaloid isolated from the marine sponge Plakortis sp. There are three plakoridines known, named plakoridine A, B, and C.
Dysidea arenaria is a species of marine sponge (poriferan) found in the Pacific Ocean. It is a member of the order Dictyoceratida, one of two sponge orders that make up the keratose or "horny" sponges in which a mineral skeleton is absent and a skeleton of organic fibers is present instead.
Mycale adhaerens, the purple scallop sponge, is a species of marine demosponge in the family Mycalidae. Mycale is a large genus and this species is placed in the subgenus Aegogropila making its full name, Mycale (Aegogropila) adhaerens. It grows symbiotically on the valves of scallop shells and is native to the west coast of North America.

Pseudoceratina is a genus of sponge within the family Pseudoceratinidae. They are characterized by possession of a dendritic fiber skeleton lacking laminar bark but containing pith. They have been found in a variety of habitats including the Great Barrier reef, the Red Sea, and Jamaica. Sponges of this genus have a microbiome known to produce a variety of chemicals that are used in pharmaceutical and anti-fouling activities. Notably, a species in this genus produces a chemical that is effective in inhibiting the migration of metastatic breast cancer cells.
Michelle Kelly, also known as Michelle Kelly-Borges, is a New Zealand scientist who specialises in sponges, their chemistry, their evolution, taxonomy, systematics, and ecology.
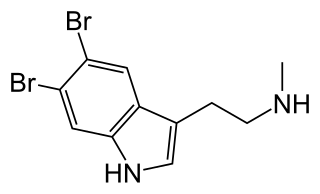
5,6-Dibromo-N-methyltryptamine (5,6-Dibromo-NMT) is a substituted tryptamine alkaloid that occurs naturally in marine sponges.

5,6-Dibromo-DMT is a substituted tryptamine alkaloid found in some marine sponges. It is briefly mentioned in Alexander Shulgin's book TiHKAL under the DMT entry and is stated to be found, along with other tryptamines, in Smenospongia aurea and other sponges.