Related Research Articles

Gram-negative bacteria are bacteria that unlike gram-positive bacteria do not retain the crystal violet stain used in the Gram staining method of bacterial differentiation. Their defining characteristic is their cell envelope, which consists of a thin peptidoglycan cell wall sandwiched between an inner (cytoplasmic) membrane and an outer membrane. These bacteria are found in all environments that support life on Earth.
Bloodstream infections (BSIs) are infections of blood caused by blood-borne pathogens. The detection of microbes in the blood is always abnormal. A bloodstream infection is different from sepsis, which is characterized by severe inflammatory or immune responses of the host organism to pathogens.
The periplasm is a concentrated gel-like matrix in the space between the inner cytoplasmic membrane and the bacterial outer membrane called the periplasmic space in gram-negative bacteria. Using cryo-electron microscopy it has been found that a much smaller periplasmic space is also present in gram-positive bacteria, between cell wall and the plasma membrane. The periplasm may constitute up to 40% of the total cell volume of gram-negative bacteria, but is a much smaller percentage in gram-positive bacteria.
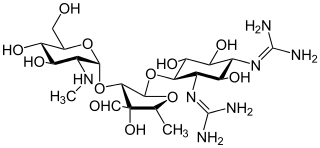
Aminoglycoside is a medicinal and bacteriologic category of traditional Gram-negative antibacterial medications that inhibit protein synthesis and contain as a portion of the molecule an amino-modified glycoside (sugar). The term can also refer more generally to any organic molecule that contains amino sugar substructures. Aminoglycoside antibiotics display bactericidal activity against Gram-negative aerobes and some anaerobic bacilli where resistance has not yet arisen but generally not against Gram-positive and anaerobic Gram-negative bacteria.

Polymyxins are antibiotics. Polymyxins B and E are used in the treatment of Gram-negative bacterial infections. They work mostly by breaking up the bacterial cell membrane. They are part of a broader class of molecules called nonribosomal peptides.

Antimicrobial peptides (AMPs), also called host defence peptides (HDPs) are part of the innate immune response found among all classes of life. Fundamental differences exist between prokaryotic and eukaryotic cells that may represent targets for antimicrobial peptides. These peptides are potent, broad spectrum antimicrobials which demonstrate potential as novel therapeutic agents. Antimicrobial peptides have been demonstrated to kill Gram negative and Gram positive bacteria, enveloped viruses, fungi and even transformed or cancerous cells. Unlike the majority of conventional antibiotics it appears that antimicrobial peptides frequently destabilize biological membranes, can form transmembrane channels, and may also have the ability to enhance immunity by functioning as immunomodulators.

Carbapenems are a class of very effective antibiotic agents most commonly used for treatment of severe bacterial infections. This class of antibiotics is usually reserved for known or suspected multidrug-resistant (MDR) bacterial infections. Similar to penicillins and cephalosporins, carbapenems are members of the beta-lactam antibiotics drug class, which kill bacteria by binding to penicillin-binding proteins, thus inhibiting bacterial cell wall synthesis. However, these agents individually exhibit a broader spectrum of activity compared to most cephalosporins and penicillins. Furthermore, carbapenems are typically unaffected by emerging antibiotic resistance, even to other beta-lactams.

Ceragenins, or cationic steroid antimicrobials (CSAs), are synthetically-produced, small-molecule chemical compounds consisting of a sterol backbone with amino acids and other chemical groups attached to them. These compounds have a net positive charge that is electrostatically attracted to the negative-charged cell membranes of certain viruses, fungi and bacteria. CSAs have a high binding affinity for such membranes and are able to rapidly disrupt the target membranes leading to rapid cell death. While CSAs have a mechanism of action that is also seen in antimicrobial peptides, which form part of the body's innate immune systum, they avoid many of the difficulties associated with their use as medicines.

Alpha defensins are a family of mammalian defensin peptides of the alpha subfamily. They are also known as cryptdins and are produced within the small bowel. Cryptdin is a portmanteau of crypt and defensin.

Class II bacteriocins are a class of small peptides that inhibit the growth of various bacteria.

Mecillinam (INN) or amdinocillin (USAN) is an extended-spectrum penicillin antibiotic of the amidinopenicillin class that binds specifically to penicillin binding protein 2 (PBP2), and is only considered to be active against Gram-negative bacteria. It is used primarily in the treatment of urinary tract infections, and has also been used to treat typhoid and paratyphoid fever. Because mecillinam has very low oral bioavailability, an orally active prodrug was developed: pivmecillinam.

Arbekacin (INN) is a semisynthetic aminoglycoside antibiotic which was derived from kanamycin. It is primarily used for the treatment of infections caused by multi-resistant bacteria including methicillin-resistant Staphylococcus aureus (MRSA). Arbekacin was originally synthesized from dibekacin in 1973 by Hamao Umezawa and collaborators. It has been registered and marketed in Japan since 1990 under the trade name Habekacin. Arbekacin is no longer covered by patent and generic versions of the drug are also available under such trade names as Decontasin and Blubatosine.
Protegrins are small peptides containing 16-18 amino acid residues. Protegrins were first discovered in porcine leukocytes and were found to have antimicrobial activity against bacteria, fungi, and some enveloped viruses. The amino acid composition of protegrins contains six positively charged arginine residues and four cysteine residues. Their secondary structure is classified as cysteine-rich β-sheet antimicrobial peptides, AMPs, that display limited sequence similarity to certain defensins and tachyplesins. In solution, the peptides fold to form an anti-parallel β-strand with the structure stabilized by two cysteine bridges formed among the four cysteine residues. Recent studies suggest that protegrins can bind to lipopolysaccharide, a property that may help them to insert into the membranes of gram-negative bacteria and permeabilize them.
Omptins are a family of bacterial proteases. They are aspartate proteases, which cleave peptides with the use of a water molecule. Found in the outer membrane of gram-negative enterobacteria such as Shigella flexneri, Yersinia pestis, Escherichia coli, and Salmonella enterica. Omptins consist of a widely conserved beta barrel spanning the membrane with 5 extracellular loops. These loops are responsible for the various substrate specificities. These proteases rely upon binding of lipopolysaccharide for activity.
Cephalosporins are a broad class of bactericidal antibiotics that include the β-lactam ring and share a structural similarity and mechanism of action with other β-lactam antibiotics. The cephalosporins have the ability to kill bacteria by inhibiting essential steps in the bacterial cell wall synthesis which in the end results in osmotic lysis and death of the bacterial cell. Cephalosporins are widely used antibiotics because of their clinical efficiency and desirable safety profile.

OmpT is an aspartyl protease found on the outer membrane of Escherichia coli. OmpT is a subtype of the family of omptin proteases, which are found on some gram-negative species of bacteria.

Finafloxacin (Xtoro) is a fluoroquinolone antibiotic. In the United States, it is approved by the Food and Drug Administration to treat acute otitis externa caused by the bacteria Pseudomonas aeruginosa and Staphylococcus aureus.
ESKAPE is an acronym comprising the scientific names of six highly virulent and antibiotic resistant bacterial pathogens including: Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp. The acronym is sometimes extended to ESKAPEE to include Escherichia coli. This group of Gram-positive and Gram-negative bacteria can evade or 'escape' commonly used antibiotics due to their increasing multi-drug resistance (MDR). As a result, throughout the world, they are the major cause of life-threatening nosocomial or hospital-acquired infections in immunocompromised and critically ill patients who are most at risk. P. aeruginosa and S. aureus are some of the most ubiquitous pathogens in biofilms found in healthcare. P. aeruginosa is a Gram-negative, rod-shaped bacterium, commonly found in the gut flora, soil, and water that can be spread directly or indirectly to patients in healthcare settings. The pathogen can also be spread in other locations through contamination, including surfaces, equipment, and hands. The opportunistic pathogen can cause hospitalized patients to have infections in the lungs, blood, urinary tract, and in other body regions after surgery. S. aureus is a Gram-positive, cocci-shaped bacterium, residing in the environment and on the skin and nose of many healthy individuals. The bacterium can cause skin and bone infections, pneumonia, and other types of potentially serious infections if it enters the body. S. aureus has also gained resistance to many antibiotic treatments, making healing difficult. Because of natural and unnatural selective pressures and factors, antibiotic resistance in bacteria usually emerges through genetic mutation or acquires antibiotic-resistant genes (ARGs) through horizontal gene transfer - a genetic exchange process by which antibiotic resistance can spread.
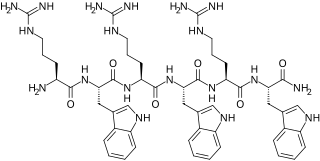
MP196 is a synthetic antimicrobial peptide. It falls under the structural class: short cationic peptides. Since it is a short cationic peptide, it can be easily synthesized, derivatized and isolated. MP196 is rich in tryptophan, a hydrophobic amino acid and arginine residues, a positively charged amino acid. It has structure: RWRWRW-NH2. This a short linear peptide with minimal pharmacophore. MP196 is effective against gram-positive bacteria and moderately effective against gram-negative bacteria.

Murepavadin also known as POL7080 is a Pseudomonas specific peptidomimetic antibiotic. It is a synthetic cyclic beta hairpin peptidomimetic based on the cationic antimicrobial peptide protegrin I (PG-1) and the first example of an outer membrane protein-targeting antibiotic class with a novel, nonlytic mechanism of action, highly active and selective against the protein transporter LptD of Pseudomonas aeruginosa. In preclinical studies the compound was highly active on a broad panel of clinical isolates including multi-drug resistant Pseudomonas bacteria with outstanding in vivo efficacy in sepsis, lung, and thigh infection models. Intravenous murepavadin is in development for the treatment of bacterial hospital-acquired pneumonia and bacterial ventilator-associated pneumonia due to Pseudomonas aeruginosa.
References
- 1 2 Neve, S., Raventós, D., et al. "NZ17074 : An Arenicin-3 variant found by HTS screening of yeast libraries" Archived October 29, 2013, at the Wayback Machine . Novozymes A/S. Copenhagen: Adenium Biotech, Web. 21 Nov. 2012.
- 1 2 Adenium Biotech Aps. EP2509617A1, 2012, "Arenicin-3 for use in the treatment of urinary tract infections"
- ↑ "Adenium Biotech" Archived October 29, 2013, at the Wayback Machine . Novozymes A/S. Copenhagen: Adenium Biotech, Web. 21 Nov. 2012.
- ↑ "Adenium Biotech". Novozymes A/S. Copenhagen: Adenium Biotech, Web. 29, Nov. 2012.
- ↑ "Arenecin" Archived 2013-06-19 at the Wayback Machine . Novozymes A/S. Copenhagen: Adenium Biotech, Web. 29, Nov. 2012.
- ↑ Lee JU, Kang DI, Zhu WL, Shin SY, Hahm KS, Kim Y (2007). "Solution structures and biological functions of the antimicrobial peptide, arenicin-1, and its linear derivative". Biopolymers. 88 (2): 208–16. doi: 10.1002/bip.20700 . PMID 17285588.
- ↑ Ovchinnikova TV, Shenkarev ZO, Nadezhdin KD, Balandin SV, Zhmak MN, Kudelina IA, Finkina EI, Kokryakov VN, Arseniev AS (August 2007). "Recombinant expression, synthesis, purification, and solution structure of arenicin". Biochemical and Biophysical Research Communications. 360 (1): 156–62. doi:10.1016/j.bbrc.2007.06.029. PMID 17585874.
- ↑ Nielsen, A.K., D. Sandvang, et al. "Transcriptional profiling indicates a dual mode-of-action of Arenicin-3" Archived October 29, 2013, at the Wayback Machine . Novozymes A/S. Copenhagen: Adenium Biotech, Web. 21 Nov. 2012.
- 1 2 Nielsen.
- ↑ Yonezawa A, Kuwahara J, Fujii N, Sugiura Y (March 1992). "Binding of tachyplesin I to DNA revealed by footprinting analysis: significant contribution of secondary structure to DNA binding and implication for biological action". Biochemistry. 31 (11): 2998–3004. doi:10.1021/bi00126a022. PMID 1372516.
- ↑ andvang, D., Neve, S., et al. "NZ17074 - Pharmacokinetic and efficacy in mice" Archived October 29, 2013, at the Wayback Machine . Novozymes A/S. Copenhagen: Adenium Biotech, Web. 21 Nov. 2012.
- ↑ Sandvang, D., Markvardsen, et al. “NZ17074 - A Novel antimicrobial peptide showing potent in vitro activity against gram negative multi-resistant clinical isolates” Archived October 29, 2013, at the Wayback Machine . Novozymes A/S. Copenhagen: Adenium Biotech, Web. 20 Nov. 2012.
- ↑ Ovchinnikova TV, Aleshina GM, Balandin SV, Krasnosdembskaya AD, Markelov ML, Frolova EI, Leonova YF, Tagaev AA, Krasnodembsky EG, Kokryakov VN (November 2004). "Purification and primary structure of two isoforms of arenicin, a novel antimicrobial peptide from marine polychaeta Arenicola marina". FEBS Letters. 577 (1–2): 209–14. doi: 10.1016/j.febslet.2004.10.012 . PMID 15527787. S2CID 23868739.