Related Research Articles

Glycoproteins are proteins which contain oligosaccharide chains covalently attached to amino acid side-chains. The carbohydrate is attached to the protein in a cotranslational or posttranslational modification. This process is known as glycosylation. Secreted extracellular proteins are often glycosylated.
A congenital disorder of glycosylation is one of several rare inborn errors of metabolism in which glycosylation of a variety of tissue proteins and/or lipids is deficient or defective. Congenital disorders of glycosylation are sometimes known as CDG syndromes. They often cause serious, sometimes fatal, malfunction of several different organ systems in affected infants. The most common sub-type is PMM2-CDG where the genetic defect leads to the loss of phosphomannomutase 2 (PMM2), the enzyme responsible for the conversion of mannose-6-phosphate into mannose-1-phosphate.

Mannose is a sugar monomer of the aldohexose series of carbohydrates. It is a C-2 epimer of glucose. Mannose is important in human metabolism, especially in the glycosylation of certain proteins. Several congenital disorders of glycosylation are associated with mutations in enzymes involved in mannose metabolism.

Luis Federico Leloir was an Argentine physician and biochemist who received the 1970 Nobel Prize in Chemistry for his discovery of the metabolic pathways by which carbohydrates are synthesized and converted into energy in the body. Although born in France, Leloir received the majority of his education at the University of Buenos Aires and was director of the private research group Fundación Instituto Campomar until his death in 1987. His research into sugar nucleotides, carbohydrate metabolism, and renal hypertension garnered international attention and led to significant progress in understanding, diagnosing and treating the congenital disease galactosemia. Leloir is buried in La Recoleta Cemetery, Buenos Aires.
Glycosylation is the reaction in which a carbohydrate, i.e. a glycosyl donor, is attached to a hydroxyl or other functional group of another molecule in order to form a glycoconjugate. In biology, glycosylation usually refers to an enzyme-catalysed reaction, whereas glycation may refer to a non-enzymatic reaction.
Dolichol refers to any of a group of long-chain mostly unsaturated organic compounds that are made up of varying numbers of isoprene units terminating in an α-saturated isoprenoid group, containing an alcohol functional group.

Oligosaccharyltransferase or OST (EC 2.4.1.119) is a membrane protein complex that transfers a 14-sugar oligosaccharide from dolichol to nascent protein. It is a type of glycosyltransferase. The sugar Glc3Man9GlcNAc2 (where Glc=Glucose, Man=Mannose, and GlcNAc=N-acetylglucosamine) is attached to an asparagine (Asn) residue in the sequence Asn-X-Ser or Asn-X-Thr where X is any amino acid except proline. This sequence is called a glycosylation sequon. The reaction catalyzed by OST is the central step in the N-linked glycosylation pathway.

The enzyme UDP-glucose 4-epimerase, also known as UDP-galactose 4-epimerase or GALE, is a homodimeric epimerase found in bacterial, fungal, plant, and mammalian cells. This enzyme performs the final step in the Leloir pathway of galactose metabolism, catalyzing the reversible conversion of UDP-galactose to UDP-glucose. GALE tightly binds nicotinamide adenine dinucleotide (NAD+), a co-factor required for catalytic activity.

Dolichyl pyrophosphate Man9GlcNAc2 alpha-1,3-glucosyltransferase is an enzyme that in humans is encoded by the ALG6 gene.

UDP-N-acetylglucosamine—dolichyl-phosphate N-acetylglucosaminephosphotransferase is an enzyme that in humans is encoded by the DPAGT1 gene.

Alpha-1,3/1,6-mannosyltransferase ALG2 is an enzyme that is encoded by the ALG2 gene. Mutations in the human gene are associated with congenital defects in glycosylation The protein encoded by the ALG2 gene belongs to two classes of enzymes: GDP-Man:Man1GlcNAc2-PP-dolichol alpha-1,3-mannosyltransferase and GDP-Man:Man2GlcNAc2-PP-dolichol alpha-1,6-mannosyltransferase.
UGGT, or UDP-glucose:glycoprotein glucosyltransferase, is a soluble enzyme resident in the lumen of the endoplasmic reticulum (ER).
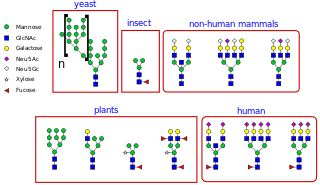
N-linked glycosylation is the attachment of an oligosaccharide, a carbohydrate consisting of several sugar molecules, sometimes also referred to as glycan, to a nitrogen atom, in a process called N-glycosylation, studied in biochemistry. The resulting protein is called an N-linked glycan, or simply an N-glycan.
O-linked glycosylation is the attachment of a sugar molecule to the oxygen atom of serine (Ser) or threonine (Thr) residues in a protein. O-glycosylation is a post-translational modification that occurs after the protein has been synthesised. In eukaryotes, it occurs in the endoplasmic reticulum, Golgi apparatus and occasionally in the cytoplasm; in prokaryotes, it occurs in the cytoplasm. Several different sugars can be added to the serine or threonine, and they affect the protein in different ways by changing protein stability and regulating protein activity. O-glycans, which are the sugars added to the serine or threonine, have numerous functions throughout the body, including trafficking of cells in the immune system, allowing recognition of foreign material, controlling cell metabolism and providing cartilage and tendon flexibility. Because of the many functions they have, changes in O-glycosylation are important in many diseases including cancer, diabetes and Alzheimer's. O-glycosylation occurs in all domains of life, including eukaryotes, archaea and a number of pathogenic bacteria including Burkholderia cenocepacia, Neisseria gonorrhoeae and Acinetobacter baumannii.
Glypiation is the addition by covalent bonding of a glycosylphosphatidylinositol (GPI) anchor and is a common post-translational modification that localizes proteins to cell membranes. This special kind of glycosylation is widely detected on surface glycoproteins in eukaryotes and some Archaea.
Dolichyl-P-Man:Man5GlcNAc2-PP-dolichol alpha-1,3-mannosyltransferase is an enzyme with systematic name dolichyl beta-D-mannosyl phosphate:D-Man-alpha-(1->2)-D-Man-alpha-(1->2)-D-Man-alpha-(1->3)-(D-Man-alpha- )-D-Man-beta-(1->4)-D-GlcNAc-beta-(1->4)-D-GlcNAc-diphosphodolichol alpha-1,3-mannosyltransferase. This enzyme catalyses the following chemical reaction

Dolichyl-P-Glc:Glc1Man9GlcNAc2-PP-dolichol alpha-1,3-glucosyltransferase is an enzyme with systematic name dolichyl beta-D-glucosyl phosphate:D-Glc-alpha-(1->3)-D-Man-alpha-(1->2)-D-Man-alpha-(1->2)-D-Man-alpha-(1->3)-(D-Man-alpha- -D-Man-alpha- - -D-Man-alpha- )-D-Man-beta-(1->4)-D-GlcNAc-beta-(1->4)-D-GlcNAc-diphosphodolichol alpha-1,3-glucosyltransferase.
Mannosyl-oligosaccharide glucosidase (MOGS) (EC 3.2.1.106, processing α-glucosidase I,Glc3Man9NAc2 oligosaccharide glucosidase, trimming glucosidase I, GCS1) is an enzyme with systematic name mannosyl-oligosaccharide glucohydrolase. MOGS is a transmembrane protein found in the membrane of the endoplasmic reticulum of eukaryotic cells. Biologically, it functions within the N-glycosylation pathway.
William Joseph Lennarz was a biochemist at Stony Brook University. He was born in May 1934 in New York City. Before Lennarz began his tenure at Stony Brook, he studied chemistry and organic chemistry. After working as a postdoctoral researcher at Harvard, he developed an interest in biochemistry. He has focused the majority of his research on biochemical processes in cells.
Rosa Muchnik de Lederkremer is an Argentine chemist. A doctor in chemical sciences, emeritus professor at the University of Buenos Aires (UBA), and senior researcher at the National Scientific and Technical Research Council (CONICET), she has received Konex Awards in the area of Organic Chemistry in 1983 and 2013. Her research includes major contributions in the area of glycobiology through investigating the inhibition of the key enzyme for the survival of Trypanosoma cruzi in the human body.
References
- 1 2 Parodi, Armando J. (2007). "How I became a biochemist". IUBMB Life. 59 (4–5): 361–363. doi: 10.1080/15216540601080198 . ISSN 1521-6551. S2CID 86051162.
- 1 2 Kresge, Nicole; Simoni, Robert D.; Hill, Robert L. (2008-04-18). "The Transient Glucosylation of Glycoproteins: the Work of Armando J. Parodi". Journal of Biological Chemistry. 283 (16): e8. ISSN 0021-9258.
- ↑ Doyle, Patricia; de la Canal, Laura; Engel, Juan C.; Parodi, Armando J. (October 1986). "Characterization of the mechanism of protein glycosylation and the structure of glycoconjugates in tissue culture trypomastigotes and intracellular amastigotes of Trypanosoma cruzi". Molecular and Biochemical Parasitology. 21 (1): 93–101. doi:10.1016/0166-6851(86)90083-6. ISSN 0166-6851. PMID 3534566.
- 1 2 "Armando J. Parodi: 2011 Karl Meyer Award Goes to Armando Parodi". Society for Glycobiology. Archived from the original on 2017-07-03. Retrieved March 8, 2020.
- ↑ Gallo, Giovanna L.; Valko, Ayelén; Aramburu, Sofía I.; Etchegaray, Emiliana; Völker, Christof; Parodi, Armando J.; D'Alessio, Cecilia (2018-11-02). "Abrogation of glucosidase I–mediated glycoprotein deglucosylation results in a sick phenotype in fission yeasts: Model for the human MOGS-CDG disorder". Journal of Biological Chemistry. 293 (52): 19957–19973. doi: 10.1074/jbc.ra118.004844 . ISSN 0021-9258. PMC 6311512 . PMID 30389790.
- 1 2 3 4 5 6 7 "Armando J. Parodi". Fundacion Konex. Archived from the original on 2018-03-26. Retrieved March 9, 2020.