
In immunology, autoimmunity is the system of immune responses of an organism against its own healthy cells, tissues and other normal body constituents. Any disease resulting from this type of immune response is termed an "autoimmune disease". Prominent examples include celiac disease, post-infectious IBS, diabetes mellitus type 1, Henoch–Schönlein purpura (HSP), systemic lupus erythematosus (SLE), Sjögren syndrome, eosinophilic granulomatosis with polyangiitis, Hashimoto's thyroiditis, Graves' disease, idiopathic thrombocytopenic purpura, Addison's disease, rheumatoid arthritis (RA), ankylosing spondylitis, polymyositis (PM), dermatomyositis (DM), and multiple sclerosis (MS). Autoimmune diseases are very often treated with steroids.
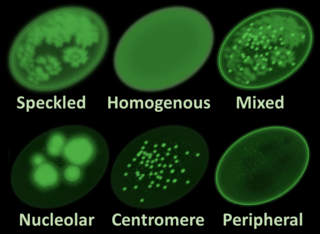
Antinuclear antibodies are autoantibodies that bind to contents of the cell nucleus. In normal individuals, the immune system produces antibodies to foreign proteins (antigens) but not to human proteins (autoantigens). In some cases, antibodies to human antigens are produced.
A connective tissue disease (collagenosis) is any disease that has the connective tissues of the body as a target of pathology. Connective tissue is any type of biological tissue with an extensive extracellular matrix that supports, binds together, and protects organs. These tissues form a framework, or matrix, for the body, and are composed of two major structural protein molecules: collagen and elastin. There are many different types of collagen protein in each of the body's tissues. Elastin has the capability of stretching and returning to its original length—like a spring or rubber band. Elastin is the major component of ligaments and skin. In patients with connective tissue disease, it is common for collagen and elastin to become injured by inflammation (ICT). Many connective tissue diseases feature abnormal immune system activity with inflammation in tissues as a result of an immune system that is directed against one's own body tissues (autoimmunity).
Mixed connective tissue disease, commonly abbreviated as MCTD, is an autoimmune disease characterized by the presence of elevated blood levels of a specific autoantibody, now called anti-U1 ribonucleoprotein (RNP) together with a mix of symptoms of systemic lupus erythematosus (SLE), scleroderma, and polymyositis. The idea behind the "mixed" disease is that this specific autoantibody is also present in other autoimmune diseases such as systemic lupus erythematosus, polymyositis, scleroderma, etc. MCTD was characterized as an individual disease in 1972 by Sharp et al., and the term was introduced by Leroy in 1980.

Bruton's tyrosine kinase, also known as tyrosine-protein kinase BTK, is a tyrosine kinase that is encoded by the BTK gene in humans. BTK plays a crucial role in B cell development.

Belimumab, sold under the brand name Benlysta, is a human monoclonal antibody that inhibits B-cell activating factor (BAFF), also known as B-lymphocyte stimulator (BLyS). It is approved in the United States and Canada, and the European Union to treat systemic lupus erythematosus and lupus nephritis.
CD22, or cluster of differentiation-22, is a molecule belonging to the SIGLEC family of lectins. It is found on the surface of mature B cells and to a lesser extent on some immature B cells. Generally speaking, CD22 is a regulatory molecule that prevents the overactivation of the immune system and the development of autoimmune diseases.
Epratuzumab is a humanized monoclonal antibody. Potential uses may be found in oncology and in treatment of inflammatory autoimmune disorders, such as systemic lupus erythematosus (SLE).

B-cell activating factor (BAFF) also known as tumor necrosis factor ligand superfamily member 13B and CD257 among other names, is a protein that in humans is encoded by the TNFSF13B gene. BAFF is also known as B Lymphocyte Stimulator (BLyS) and TNF- and APOL-related leukocyte expressed ligand (TALL-1) and the Dendritic cell-derived TNF-like molecule.

Interferon alpha-1 is a protein that in humans is encoded by the IFNA1 gene.

B-cell maturation antigen, also known as tumor necrosis factor receptor superfamily member 17 (TNFRSF17), is a protein that in humans is encoded by the TNFRSF17 gene.

An autoimmune disease is a condition that results from an anomalous response of the adaptive immune system, wherein it mistakenly targets and attacks healthy, functioning parts of the body as if they were foreign organisms. It is estimated that there are more than 80 recognized autoimmune diseases, with recent scientific evidence suggesting the existence of potentially more than 100 distinct conditions. Nearly any body part can be involved.

Anti-double stranded DNA (Anti-dsDNA) antibodies are a group of anti-nuclear antibodies (ANA) the target antigen of which is double stranded DNA. Blood tests such as enzyme-linked immunosorbent assay (ELISA) and immunofluorescence are routinely performed to detect anti-dsDNA antibodies in diagnostic laboratories. They are highly diagnostic of systemic lupus erythematosus (SLE) and are implicated in the pathogenesis of lupus nephritis.

Lupus, technically known as systemic lupus erythematosus (SLE), is an autoimmune disease in which the body's immune system mistakenly attacks healthy tissue in many parts of the body. Symptoms vary among people and may be mild to severe. Common symptoms include painful and swollen joints, fever, chest pain, hair loss, mouth ulcers, swollen lymph nodes, feeling tired, and a red rash which is most commonly on the face. Often there are periods of illness, called flares, and periods of remission during which there are few symptoms.

Tolerx, Inc. was a biopharmaceutical company headquartered in Cambridge, Massachusetts. The company was focused on discovering and developing new therapies designed to treat patients by reprogramming the immune system, allowing for long-term remission of immune-related diseases after a short course of therapy. Targeted diseases include type 1 diabetes, rheumatoid arthritis, Inflammatory bowel disease (IBD), cancer, chronic and viral diseases. In 2008, Tolerx was named one of Fierce Biotech’s Fierce 15. In October 2011, Tolerx was shut down due to an unsuccessful Phase III trial in patients recently diagnosed with Type 1 diabetes.
Anti-histone antibodies are autoantibodies that are a subset of the anti-nuclear antibody family, which specifically target histone protein subunits or histone complexes. They were first reported by Henry Kunkel, H.R. Holman, and H.R.G. Dreicher in their studies of cellular causes of lupus erythematosus in 1959–60. Today, anti-histone antibodies are still used as a marker for systemic lupus erythematosus, but are also implicated in other autoimmune diseases like Sjögren syndrome, dermatomyositis, or rheumatoid arthritis. Anti-histone antibodies can be used as a marker for drug-induced lupus.

Anti-SSA autoantibodies are a type of anti-nuclear autoantibodies that are associated with many autoimmune diseases, such as systemic lupus erythematosus (SLE), SS/SLE overlap syndrome, subacute cutaneous lupus erythematosus (SCLE), neonatal lupus and primary biliary cirrhosis. They are often present in Sjögren's syndrome (SS). Additionally, Anti-Ro/SSA can be found in other autoimmune diseases such as systemic sclerosis (SSc), polymyositis/dermatomyositis (PM/DM), rheumatoid arthritis (RA), and mixed connective tissue disease (MCTD), and are also associated with heart arrhythmia.
Blisibimod is a selective antagonist of B-cell activating factor, being developed by Anthera Pharmaceuticals as a treatment for systemic lupus erythematosus. It is currently under active investigation in clinical trials.
Ianalumab is a monoclonal antibody that is being investigated for autoimmune hepatitis, multiple sclerosis, pemphigus vulgaris, rheumatoid arthritis, Sjögren syndrome, and systemic lupus erythematosus.
Telitacicept is a pharmaceutical drug used for the treatment of systemic lupus erythematosus (SLE). It is being developed by Yantai Rongchang Pharmaceuticals and RemeGen for various autoimmune diseases. In 2021, telitacicept was approved in China for the treatment of patients with active SLE. It is also being studied for the treatment of rheumatoid arthritis (RA) and multiple sclerosis (MS).











