
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms, when they share electrons, is known as covalent bonding. For many molecules, the sharing of electrons allows each atom to attain the equivalent of a full valence shell, corresponding to a stable electronic configuration. In organic chemistry, covalent bonding is much more common than ionic bonding.

In crystallography, crystal structure is a description of ordered arrangement of atoms, ions, or molecules in a crystalline material. Ordered structures occur from intrinsic nature of constituent particles to form symmetric patterns that repeat along the principal directions of three-dimensional space in matter.
The van der Waals radius, rw, of an atom is the radius of an imaginary hard sphere representing the distance of closest approach for another atom. It is named after Johannes Diderik van der Waals, winner of the 1910 Nobel Prize in Physics, as he was the first to recognise that atoms were not simply points and to demonstrate the physical consequences of their size through the van der Waals equation of state.
Matter waves are a central part of the theory of quantum mechanics, being half of wave–particle duality. At all scales where measurements have been practical, matter exhibits wave-like behavior. For example, a beam of electrons can be diffracted just like a beam of light or a water wave.
In many areas of science, Bragg's law, Wulff–Bragg's condition, or Laue–Bragg interference are a special case of Laue diffraction, giving the angles for coherent scattering of waves from a large crystal lattice. It describes how the superposition of wave fronts scattered by lattice planes leads to a strict relation between the wavelength and scattering angle. This law was initially formulated for X-rays, but it also applies to all types of matter waves including neutron and electron waves if there are a large number of atoms, as well as visible light with artificial periodic microscale lattices.
In optics, the Fraunhofer diffraction equation is used to model the diffraction of waves when plane waves are incident on a diffracting object, and the diffraction pattern is viewed at a sufficiently long distance from the object, and also when it is viewed at the focal plane of an imaging lens. In contrast, the diffraction pattern created near the diffracting object and is given by the Fresnel diffraction equation.
Amorphous carbon is free, reactive carbon that has no crystalline structure. Amorphous carbon materials may be stabilized by terminating dangling-π bonds with hydrogen. As with other amorphous solids, some short-range order can be observed. Amorphous carbon is often abbreviated to aC for general amorphous carbon, aC:H or HAC for hydrogenated amorphous carbon, or to ta-C for tetrahedral amorphous carbon.
In chemistry, bond energy (BE) is one measure of the strength of a chemical bond. It is sometimes called the mean bond, bond enthalpy, average bond enthalpy, or bond strength. IUPAC defines bond energy as the average value of the gas-phase bond-dissociation energy for all bonds of the same type within the same chemical species.
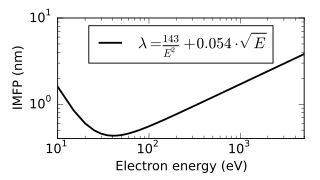
The inelastic mean free path (IMFP) is an index of how far an electron on average travels through a solid before losing energy.
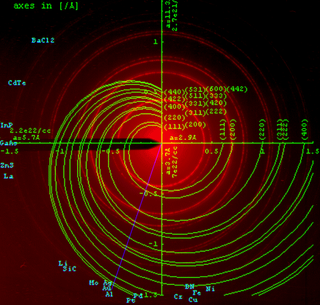
Powder diffraction is a scientific technique using X-ray, neutron, or electron diffraction on powder or microcrystalline samples for structural characterization of materials. An instrument dedicated to performing such powder measurements is called a powder diffractometer.
In molecular geometry, bond length or bond distance is defined as the average distance between nuclei of two bonded atoms in a molecule. It is a transferable property of a bond between atoms of fixed types, relatively independent of the rest of the molecule.
In solid-state physics, the tight-binding model is an approach to the calculation of electronic band structure using an approximate set of wave functions based upon superposition of wave functions for isolated atoms located at each atomic site. The method is closely related to the LCAO method used in chemistry. Tight-binding models are applied to a wide variety of solids. The model gives good qualitative results in many cases and can be combined with other models that give better results where the tight-binding model fails. Though the tight-binding model is a one-electron model, the model also provides a basis for more advanced calculations like the calculation of surface states and application to various kinds of many-body problem and quasiparticle calculations.
In chemistry, stacking refers to superposition of molecules or atomic sheets owing to attractive interactions between these molecules or sheets.
The Hückel method or Hückel molecular orbital theory, proposed by Erich Hückel in 1930, is a simple method for calculating molecular orbitals as linear combinations of atomic orbitals. The theory predicts the molecular orbitals for π-electrons in π-delocalized molecules, such as ethylene, benzene, butadiene, and pyridine. It provides the theoretical basis for Hückel's rule that cyclic, planar molecules or ions with π-electrons are aromatic. It was later extended to conjugated molecules such as pyridine, pyrrole and furan that contain atoms other than carbon and hydrogen (heteroatoms). A more dramatic extension of the method to include σ-electrons, known as the extended Hückel method (EHM), was developed by Roald Hoffmann. The extended Hückel method gives some degree of quantitative accuracy for organic molecules in general and was used to provide computational justification for the Woodward–Hoffmann rules. To distinguish the original approach from Hoffmann's extension, the Hückel method is also known as the simple Hückel method (SHM).
In crystallography, atomic packing factor (APF), packing efficiency, or packing fraction is the fraction of volume in a crystal structure that is occupied by constituent particles. It is a dimensionless quantity and always less than unity. In atomic systems, by convention, the APF is determined by assuming that atoms are rigid spheres. The radius of the spheres is taken to be the maximum value such that the atoms do not overlap. For one-component crystals (those that contain only one type of particle), the packing fraction is represented mathematically by
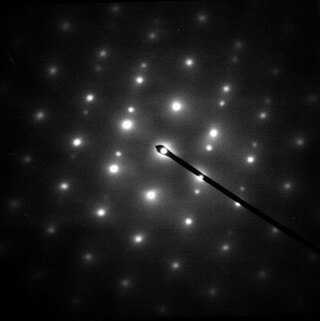
Selected area (electron) diffraction is a crystallographic experimental technique typically performed using a transmission electron microscope (TEM). It is a specific case of electron diffraction used primarily in material science and solid state physics as one of the most common experimental techniques. Especially with appropriate analytical software, SAD patterns (SADP) can be used to determine crystal orientation, measure lattice constants or examine its defects.
Melting-point depression is the phenomenon of reduction of the melting point of a material with a reduction of its size. This phenomenon is very prominent in nanoscale materials, which melt at temperatures hundreds of degrees lower than bulk materials.
Free spectral range (FSR) is the spacing in optical frequency or wavelength between two successive reflected or transmitted optical intensity maxima or minima of an interferometer or diffractive optical element.
The Mott–Bethe formula is an approximation used to calculate atomic electron scattering form factors, , from atomic X-ray scattering form factors, . The formula was derived independently by Hans Bethe and Neville Mott both in 1930, and simply follows from applying the first Born approximation for the scattering of electrons via the Coulomb interaction together with the Poisson equation for the charge density of an atom in the Fourier domain. Following the first Born approximation,

The scanning helium microscope (SHeM) is a form of microscopy that uses low-energy (5–100 meV) neutral helium atoms to image the surface of a sample without any damage to the sample caused by the imaging process. Since helium is inert and neutral, it can be used to study delicate and insulating surfaces. Images are formed by rastering a sample underneath an atom beam and monitoring the flux of atoms that are scattered into a detector at each point.










