
A tyrosine kinase is an enzyme that can transfer a phosphate group from ATP to the tyrosine residues of specific proteins inside a cell. It functions as an "on" or "off" switch in many cellular functions.
Vascular endothelial growth factor, originally known as vascular permeability factor (VPF), is a signal protein produced by many cells that stimulates the formation of blood vessels. To be specific, VEGF is a sub-family of growth factors, the platelet-derived growth factor family of cystine-knot growth factors. They are important signaling proteins involved in both vasculogenesis and angiogenesis.

Sunitinib, sold under the brand name Sutent, is an anti-cancer medication. It is a small-molecule, multi-targeted receptor tyrosine kinase (RTK) inhibitor that was approved by the FDA for the treatment of renal cell carcinoma (RCC) and imatinib-resistant gastrointestinal stromal tumor (GIST) in January 2006. Sunitinib was the first cancer drug simultaneously approved for two different indications.

Receptor tyrosine kinases (RTKs) are the high-affinity cell surface receptors for many polypeptide growth factors, cytokines, and hormones. Of the 90 unique tyrosine kinase genes identified in the human genome, 58 encode receptor tyrosine kinase proteins. Receptor tyrosine kinases have been shown not only to be key regulators of normal cellular processes but also to have a critical role in the development and progression of many types of cancer. Mutations in receptor tyrosine kinases lead to activation of a series of signalling cascades which have numerous effects on protein expression. Receptor tyrosine kinases are part of the larger family of protein tyrosine kinases, encompassing the receptor tyrosine kinase proteins which contain a transmembrane domain, as well as the non-receptor tyrosine kinases which do not possess transmembrane domains.
Matuzumab is a humanized monoclonal antibody for the treatment of cancer. It binds to the epidermal growth factor receptor (EGFR) with high affinity. The mouse monoclonal antibody (mAb425) from which matuzumab was developed at the Wistar Institute in Philadelphia, Pennsylvania

Semaxanib is a tyrosine-kinase inhibitor drug designed by SUGEN as a cancer therapeutic. It is an experimental stage drug, not licensed for use on human patients outside clinical trials. Semaxanib is a potent and selective synthetic inhibitor of the Flk-1/KDR vascular endothelial growth factor (VEGF) receptor tyrosine kinase. It targets the VEGF pathway, and both in vivo and in vitro studies have demonstrated antiangiogenic potential.

John Kuriyan is the dean of basic sciences and a professor of biochemistry at Vanderbilt University School of Medicine. He was formerly the Chancellor's Professor at the University of California, Berkeley in the departments of molecular and cell biology (MCB) and chemistry, a faculty scientist in Berkeley Lab's physical biosciences division, and a Howard Hughes Medical Institute investigator. He is a member of the National Academy of Sciences and he has also been on the Life Sciences jury for the Infosys Prize in 2009, 2019 and 2020.

VEGF receptors (VEGFRs) are receptors for vascular endothelial growth factor (VEGF). There are three main subtypes of VEGFR, numbered 1, 2 and 3. Depending on alternative splicing, they may be membrane-bound (mbVEGFR) or soluble (sVEGFR).
A growth factor receptor is a receptor that binds to a growth factor. Growth factor receptors are the first stop in cells where the signaling cascade for cell differentiation and proliferation begins. Growth factors, which are ligands that bind to the receptor are the initial step to activating the growth factor receptors and tells the cell to grow and/or divide.

Alexander Levitzki is an Israeli biochemist who is a professor of biochemistry at the Alexander Silberman Institute of Life Sciences, the Hebrew University of Jerusalem.
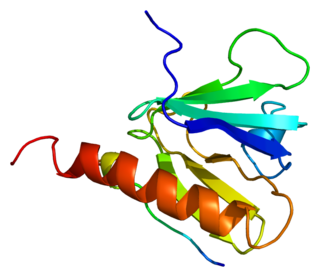
Insulin receptor substrate 1 (IRS-1) is a signaling adapter protein that in humans is encoded by the IRS-1 gene. It is a 131 kDa protein with amino acid sequence of 1242 residues. It contains a single pleckstrin homology (PH) domain at the N-terminus and a PTB domain ca. 40 residues downstream of this, followed by a poorly conserved C-terminus tail. Together with IRS2, IRS3 (pseudogene) and IRS4, it is homologous to the Drosophila protein chico, whose disruption extends the median lifespan of flies up to 48%. Similarly, Irs1 mutant mice experience moderate life extension and delayed age-related pathologies.
SUGEN (Sugen) was a drug discovery company focused on development of protein kinase inhibitors. It was founded in 1991, and shut down in 2003, after pioneering protein kinases as therapeutic targets and developing the successful cancer therapy sunitinib (Sutent).

Toceranib is a receptor tyrosine kinase inhibitor and is used in the treatment of canine mast cell tumor also called mastocytoma. Together with masitinib, toceranib is the only dog-specific anti-cancer drug approved by the U.S. Food and Drug Administration (FDA). It is sold under the brand name Palladia as its phosphate salt, toceranib phosphate (INN) by Pfizer. It was developed by SUGEN as SU11654, a sister compound to sunitinib, which was later approved for human therapies. Toceranib is likely to act mostly through inhibition of the kit tyrosine kinase, though it may also have an anti-angiogenic effect.
Joseph Schlessinger is a Yugoslav-born Israeli-American biochemist and biophysician. He is chair of the Pharmacology Department at Yale University School of Medicine in New Haven, Connecticut, as well as the founding director of the school's new Cancer Biology Institute. His area of research is signaling through tyrosine phosphorylation, which is important in many areas of cellular regulation, especially growth control and cancer. Schlessinger's work has led to an understanding of the mechanism of transmembrane signaling by receptor tyrosine kinases and how the resulting signals control cell growth and differentiation.

A tyrosine kinase inhibitor (TKI) is a pharmaceutical drug that inhibits tyrosine kinases. Tyrosine kinases are enzymes responsible for the activation of many proteins by signal transduction cascades. The proteins are activated by adding a phosphate group to the protein (phosphorylation), a step that TKIs inhibit. TKIs are typically used as anticancer drugs. For example, they have substantially improved outcomes in chronic myelogenous leukemia. They have also been used to treat other diseases, such as idiopathic pulmonary fibrosis.
Foretinib is an experimental drug candidate for the treatment of cancer. It was discovered by Exelixis and is under development by GlaxoSmithKline. About 10 Phase II clinical trials have been run. As of October 2015 it appears development has been discontinued.
Dalotuzumab is an anti-IGF1 receptor (IGF1R) humanized monoclonal antibody designed for the potential treatment of various cancers. Common adverse effects include hyperglycemia, nausea, vomiting, and fatigue. Dalotuzumab was developed by Merck and Co., Inc.

Brivanib alaninate (INN/USAN) also known as BMS-582664 is an investigational, anti-tumorigenic drug for oral administration. The drug is being developed by Bristol-Myers Squibb for the treatment of hepatocellular carcinoma or HCC, the most common type of liver cancer. Brivanib is no longer in active development.

György Kéri was a Hungarian biochemist, professor and Doctor of Biological Sciences (D.Sc.). His major field of research was signal transduction therapy and he participated in the development of novel drug discovery technologies and drug candidates that entered the clinical development process.
VEGFR-2 inhibitor, also known as kinase insert domain receptor(KDR) inhibitor, are tyrosine kinase receptor inhibitors that reduce angiogenesis or lymphangiogenesis, leading to anticancer activity. Generally they are small, synthesised molecules that bind competitively to the ATP-site of the tyrosine kinase domain. VEGFR-2 selective inhibitor can interrupt multiple signaling pathways involved in tumor, including proliferation, metastasis and angiogenesis.











