
Oxidative phosphorylation or electron transport-linked phosphorylation or terminal oxidation is the metabolic pathway in which cells use enzymes to oxidize nutrients, thereby releasing the chemical energy stored within the nutrients in order to produce adenosine triphosphate (ATP). In eukaryotes, this takes place inside mitochondria. Almost all aerobic organisms carry out oxidative phosphorylation. This pathway is so pervasive because it releases more energy than alternative fermentation processes such as anaerobic glycolysis.

Escherichia coli, also known as E. coli, is a Gram-negative, facultative anaerobic, rod-shaped, coliform bacterium of the genus Escherichia that is commonly found in the lower intestine of warm-blooded organisms (endotherms). Most E. coli strains are harmless, but some serotypes (EPEC, ETEC etc.) can cause serious food poisoning in their hosts, and are occasionally responsible for food contamination incidents that prompt product recalls. The harmless strains are part of the normal microbiota of the gut, and can benefit their hosts by producing vitamin K2, (which helps blood to clot) and preventing colonisation of the intestine with pathogenic bacteria, having a mutualistic relationship. E. coli is expelled into the environment within fecal matter. The bacterium grows massively in fresh fecal matter under aerobic conditions for 3 days, but its numbers decline slowly afterwards.

Hypochlorous acid (HOCl or HClO) is a weak acid that forms when chlorine dissolves in water, and itself partially dissociates, forming hypochlorite, ClO−. HClO and ClO− are oxidizers, and the primary disinfection agents of chlorine solutions. HClO cannot be isolated from these solutions due to rapid equilibration with its precursor. Sodium hypochlorite (NaClO) and calcium hypochlorite (Ca(ClO)2), are bleaches, deodorants, and disinfectants.

Alkaline phosphatase, or basic phosphatase, is a homodimeric protein enzyme of 86 kilodaltons. Each monomer contains five cysteine residues, two zinc atoms and one magnesium atom crucial to its catalytic function, and it is optimally active at alkaline pH environments.
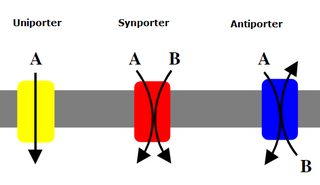
An antiporter (also called exchanger or counter-transporter) is a cotransporter and integral membrane protein involved in secondary active transport of two or more different molecules or ions across a phospholipid membrane such as the plasma membrane in opposite directions, one into the cell and one out of the cell. Na+/H+ antiporters have been reviewed.
Magnesium transporters are proteins that transport magnesium across the cell membrane. All forms of life require magnesium, yet the molecular mechanisms of Mg2+ uptake from the environment and the distribution of this vital element within the organism are only slowly being elucidated.
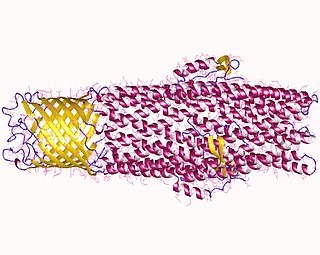
All microorganisms, with a few exceptions, have highly conserved DNA sequences in their genome that are transcribed and translated to efflux pumps. Efflux pumps are capable of moving a variety of different toxic compounds out of cells, such as antibiotics, heavy metals, organic pollutants, plant-produced compounds, quorum sensing signals, bacterial metabolites and neurotransmitters via active efflux, which is vital part for xenobiotic metabolism. This active efflux mechanism is responsible for various types of resistance to bacterial pathogens within bacterial species - the most concerning being antibiotic resistance because microorganisms can have adapted efflux pumps to divert toxins out of the cytoplasm and into extracellular media.

Oxaloacetate decarboxylase is a carboxy-lyase involved in the conversion of oxaloacetate into pyruvate.
The galactose permease or GalP found in Escherichia coli is an integral membrane protein involved in the transport of monosaccharides, primarily hexoses, for utilization by E. coli in glycolysis and other metabolic and catabolic pathways (3,4). It is a member of the Major Facilitator Super Family (MFS) and is homologue of the human GLUT1 transporter (4). Below you will find descriptions of the structure, specificity, effects on homeostasis, expression, and regulation of GalP along with examples of several of its homologues.

The heat shock proteins HslV and HslU are expressed in many bacteria such as E. coli in response to cell stress. The hslV protein is a protease and the hslU protein is an ATPase; the two form a symmetric assembly of four stacked rings, consisting of an hslV dodecamer bound to an hslU hexamer, with a central pore in which the protease and ATPase active sites reside. The hslV protein degrades unneeded or damaged proteins only when in complex with the hslU protein in the ATP-bound state. HslV is thought to resemble the hypothetical ancestor of the proteasome, a large protein complex specialized for regulated degradation of unneeded proteins in eukaryotes, many archaea, and a few bacteria. HslV bears high similarity to core subunits of proteasomes.

Maltose-binding protein (MBP) is a part of the maltose/maltodextrin system of Escherichia coli, which is responsible for the uptake and efficient catabolism of maltodextrins. It is a complex regulatory and transport system involving many proteins and protein complexes. MBP has an approximate molecular mass of 42.5 kilodaltons.
Translocase is a general term for a protein that assists in moving another molecule, usually across a cell membrane. These enzymes catalyze the movement of ions or molecules across membranes or their separation within membranes. The reaction is designated as a transfer from “side 1” to “side 2” because the designations “in” and “out”, which had previously been used, can be ambiguous. Translocases are the most common secretion system in Gram positive bacteria.
In enzymology, a phosphate-transporting ATPase (EC 3.6.3.27) is an enzyme that catalyzes the chemical reaction
In enzymology, a polyamine-transporting ATPase (EC 3.6.3.31) is an enzyme that catalyzes the chemical reaction
In enzymology, a sulfate-transporting ATPase (EC 3.6.3.25) is an enzyme that catalyzes the chemical reaction
In molecular biology, the cytochrome c assembly protein family includes various proteins involved in cytochrome c assembly from mitochondria and bacteria. Members of this family include: CycK from Rhizobium leguminosarum, CcmC from Escherichia coli and Paracoccus denitrificans, and orf240 from Triticum aestivum (Wheat) mitochondria. The members of this family are probably integral membrane proteins with six predicted transmembrane helices that may comprise the membrane component of an ABC transporter complex. This transporter may be necessary for transport of some component needed for cytochrome c assembly. One member, R. leguminosarum CycK, contains a putative haem-binding motif. Wheat orf240 also contains a putative haem-binding motif and is a proposed ABC transporter with c-type haem as its proposed substrate. However it seems unlikely that all members of this family transport haem or c-type apocytochromes because P. denitrificans CcmC transports neither.

Ammonia transporters are structurally related membrane transport proteins called Amt proteins in bacteria and plants, methylammonium/ammonium permeases (MEPs) in yeast, or Rhesus (Rh) proteins in chordates. In humans, the RhAG, RhBG, and RhCG Rhesus proteins constitute solute carrier family 42 whilst RhD and RhCE form the Rh blood group system. The three-dimensional structure of the ammonia transport protein AmtB from Escherichia coli has been determined by x-ray crystallography revealing a hydrophobic ammonia channel. The human RhCG ammonia transporter was found to have a similar ammonia-conducting channel structure. It was proposed that the erythrocyte Rh complex is a heterotrimer of RhAG, RhD, and RhCE subunits in which RhD and RhCE might play roles in anchoring the ammonia-conducting RhAG subunit to the cytoskeleton. Based on reconstitution experiments, purified RhCG subunits alone can function to transport ammonia. RhCG is required for normal acid excretion by the mouse kidney and epididymis.
The potassium (K+) uptake permease (KUP) family (TC# 2.A.72) is a member of the APC superfamily of secondary carriers. Proteins of the KUP/HAK/KT family include the KUP (TrkD) protein of E. coli and homologues in both Gram-positive and Gram-negative bacteria. High affinity (20 μM) K+ uptake systems (Hak1, TC# 2.A.72.2.1) of the yeast Debaryomyces occidentalis as well as the fungus, Neurospora crassa, and several homologues in plants have been characterized. Arabidopsis thaliana and other plants possess multiple KUP family paralogues. While many plant proteins cluster tightly together, the Hak1 proteins from yeast as well as the two Gram-positive and Gram-negative bacterial proteins are distantly related on the phylogenetic tree for the KUP family. All currently classified members of the KUP family can be found in the Transporter Classification Database.

ZnuABC is a high-affinity transporter specialized for transporting zinc ions as part of a system for metal ion homeostasis in bacteria. The complex is a member of the ATP-binding cassette (ABC) transporter protein family. The transporter contains three protein components:
The Monovalent Cation:Proton Antiporter-2 (CPA2) Family is a moderately large family of transporters belonging to the CPA superfamily. Members of the CPA2 family have been found in bacteria, archaea and eukaryotes. The proteins of the CPA2 family consist of between 333 and 900 amino acyl residues and exhibit 10-14 transmembrane α-helical spanners (TMSs).











