Immunotherapy or biological therapy is the treatment of disease by activating or suppressing the immune system. Immunotherapies designed to elicit or amplify an immune response are classified as activation immunotherapies, while immunotherapies that reduce or suppress are classified as suppression immunotherapies. Immunotherapy is under preliminary research for its potential to treat various forms of cancer.

Tumor necrosis factor (TNF), formerly known as TNF-α, is a chemical messenger produced by the immune system that induces inflammation. TNF is produced primarily by activated macrophages, and induces inflammation by binding to its receptors on other cells. It is a member of the tumor necrosis factor superfamily, a family of transmembrane proteins that are cytokines, chemical messengers of the immune system. Excessive production of TNF plays a critical role in several inflammatory diseases, and TNF-blocking drugs are often employed to treat these diseases.

Cluster of differentiation 40, CD40 is a type I transmembrane protein found on antigen-presenting cells and is required for their activation. The binding of CD154 (CD40L) on TH cells to CD40 activates antigen presenting cells and induces a variety of downstream effects.
Lymphotoxin is a member of the tumor necrosis factor (TNF) superfamily of cytokines, whose members are responsible for regulating the growth and function of lymphocytes and are expressed by a wide variety of cells in the body.

Tumor necrosis factor ligand superfamily member 9 also known as 4-1BB ligand or 4-1BBL or CD137L is a protein that in humans is encoded by the TNFSF9 gene.

CD137, a member of the tumor necrosis factor (TNF) receptor family, is a type 1 transmembrane protein, expressed on surfaces of leukocytes and non-immune cells. Its alternative names are tumor necrosis factor receptor superfamily member 9 (TNFRSF9), 4-1BB, and induced by lymphocyte activation (ILA). It is of interest to immunologists as a co-stimulatory immune checkpoint molecule, and as a potential target in cancer immunotherapy.

Toll-like receptor 5, also known as TLR5, is a protein which in humans is encoded by the TLR5 gene. It is a member of the toll-like receptor (TLR) family. TLR5 is known to recognize bacterial flagellin from invading mobile bacteria. It has been shown to be involved in the onset of many diseases, including Inflammatory bowel disease due to the high expression of TLR in intestinal lamina propria dendritic cells. Recent studies have also shown that malfunctioning of TLR5 is likely related to rheumatoid arthritis, osteoclastogenesis, and bone loss. Abnormal TLR5 functioning is related to the onset of gastric, cervical, endometrial and ovarian cancers.

Programmed cell death protein 1(PD-1),. PD-1 is a protein encoded in humans by the PDCD1 gene. PD-1 is a cell surface receptor on T cells and B cells that has a role in regulating the immune system's response to the cells of the human body by down-regulating the immune system and promoting self-tolerance by suppressing T cell inflammatory activity. This prevents autoimmune diseases, but it can also prevent the immune system from killing cancer cells.

B- and T-lymphocyte attenuator or BTLA is a protein that belongs to the CD28 immunoglobulin superfamily (IgSF) which is encoded by the BTLA gene located on the 3rd human chromosome. BTLA was first discovered in 2003 as an inhibitor of Th1 expansion and it became the 3rd member of the CD28 IgSF. However, its discovered ligand herpes virus entry mediator or HVEM belongs to the tumor necrosis factor receptor superfamily (TNFRSF). This finding was surprising because until the discovery of HVEM it was believed that receptors and ligands always belong to the same family.
A macrophage-activating factor (MAF) is a lymphokine or other receptor based signal that primes macrophages towards cytotoxicity to tumors, cytokine secretion, or clearance of pathogens. Similar molecules may cause development of an inhibitory, regulatory phenotype. A MAF can also alter the ability of macrophages to present MHC I antigen, participate in Th responses, and/or affect other immune responses.
Urelumab is a fully human, non‐ligand binding, CD137 agonist immunoglobulin‐γ 4 (IgG4) monoclonal antibody. It was developed utilizing Medarex's UltiMAb(R) technology by Bristol-Myers Squibb for the treatment of cancer and solid tumors. Urelumab promotes anti-tumor immunity, or an immune response against tumor cells, via CD137 activation. The application of Urelumab has been limited because it can cause severe liver toxicity.
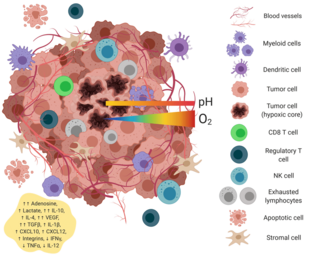
The tumor microenvironment is a complex ecosystem surrounding a tumor, composed of cancer cells, stromal tissue and the extracellular matrix. Mutual interaction between cancer cells and the different components of the tumor microenvironment support its growth and invasion in healthy tissues which correlates with tumor resistance to current treatments and poor prognosis. The tumor microenvironment is in constant change because of the tumor's ability to influence the microenvironment by releasing extracellular signals, promoting tumor angiogenesis and inducing peripheral immune tolerance, while the immune cells in the microenvironment can affect the growth and evolution of cancerous cells.
Myeloid-derived suppressor cells (MDSC) are a heterogeneous group of immune cells from the myeloid lineage.

Immune checkpoints are regulators of the immune system. These pathways are crucial for self-tolerance, which prevents the immune system from attacking cells indiscriminately. However, some cancers can protect themselves from attack by stimulating immune checkpoint targets.
Clostridium novyi-NT is an attenuated strain of Clostridium novyi that is under investigation as a cancer treatment. It is one of several pathogenic species of Clostridium bacteria that have been examined for this purpose. The modification eliminated the secretion of α-toxin.
The dendritic cell-based cancer vaccine is an innovation in therapeutic strategy for cancer patients.
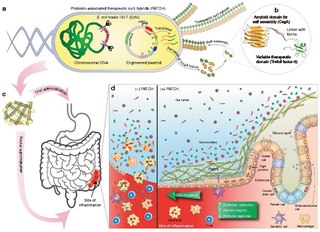
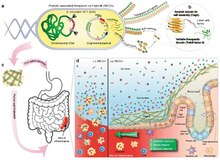
A living medicine is a type of biologic that consists of a living organism that is used to treat a disease. This usually takes the form of a cell or a virus that has been genetically engineered to possess therapeutic properties that is injected into a patient. Perhaps the oldest use of a living medicine is the use of leeches for bloodletting, though living medicines have advanced tremendously since that time.
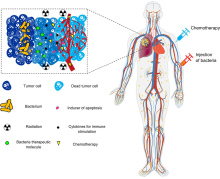
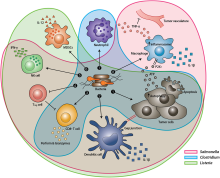
Tumor-homing bacteria are facultative or obligate anaerobic bacteria that are able to target cancerous cells in the body, suppress tumor growth and survive in the body for a long time even after the infection. When this type of bacteria is administered into the body, it migrates to the cancerous tissues and starts to grow, and then deploys distinct mechanisms to destroy solid tumors. Each bacteria species uses a different process to eliminate the tumor. Some common tumor homing bacteria include Salmonella, Clostridium, Bifidobacterium, Listeria, and Streptococcus. The earliest research of this type of bacteria was highlighted in 1813 when scientists began observing that patients that had gas gangrene, an infection caused by the bacteria Clostridium, were able to have tumor regressions.
Laurence Zitvogel is a French physician-scientist specializing in oncology and immunology. Zitvogel is a clinical oncologist, a researcher in the Laboratory of Tumor Immunology and Immunotherapy, and a professor at Paris-Saclay University. She has studied the correlation between the immune system and the success of cancer treatments for over 30 years. Her primary research experience lies in exosomes, studying the biological impact of structural abnormalities on malignant neoplasms, and anti-tumor therapy. Through her work as a professor and researcher, Zitvogel discovered that chemotherapy could delay the growth of tumors in mouse models. Her team reported the first anticancer probiotic, Enterococcus hirae. As of 2020, she is researching an effective and inexpensive diagnostic test to predict dysbiosis and is investigating the promising lead on the role of gut microbiotes in anti-tumour immunotherapy.
RNA therapeutics are a new class of medications based on ribonucleic acid (RNA). Research has been working on clinical use since the 1990s, with significant success in cancer therapy in the early 2010s. In 2020 and 2021, mRNA vaccines have been developed globally for use in combating the coronavirus disease. The Pfizer–BioNTech COVID-19 vaccine was the first mRNA vaccine approved by a medicines regulator, followed by the Moderna COVID-19 vaccine, and others.