This article has multiple issues. Please help improve it or discuss these issues on the talk page . (Learn how and when to remove these template messages) |
Barbara Stuart is a specialist in spectroscopy.
This article has multiple issues. Please help improve it or discuss these issues on the talk page . (Learn how and when to remove these template messages) |
Barbara Stuart is a specialist in spectroscopy.
Stuart studied her B.Sc. at the University of Sydney in 1987, tutoring at the university for 3 years, then studied a M.Sc. biophysical chemistry, graduating in 1990. [1] Stuart then moved to the UK and studied a PhD in polymer engineering at Imperial College London, graduating in 1993. [1] Stuart then began lecturing in Physical Chemistry at the University of Greenwich for 2 years before returning to Australia to lecture at the University of Technology Sydney. [2]
Stuart has published Biological Applications of Infrared Spectroscopy in 1997, [3] Infrared Spectroscopy: Fundamentals and Applications in 2004, [4] Analytical techniques in materials conservation in 2007 [5] Polymer Analysis in 2008, [6] and Forensic Analytical Techniques in 2012. [7]

Analytical chemistry studies and uses instruments and methods used to separate, identify, and quantify matter. In practice, separation, identification or quantification may constitute the entire analysis or be combined with another method. Separation isolates analytes. Qualitative analysis identifies analytes, while quantitative analysis determines the numerical amount or concentration. Analytical chemistry is the science of obtaining, processing, and communicating information about the composition and structure of matter. In other words, it is the art and science of determining what matter is and how much of it exists. It is one of the most popular fields of work for ACS chemists.

Infrared spectroscopy is the measurement of the interaction of infrared radiation with matter by absorption, emission, or reflection. It is used to study and identify chemical substances or functional groups in solid, liquid, or gaseous forms. It can be used to characterize new materials or identify and verify known and unknown samples. The method or technique of infrared spectroscopy is conducted with an instrument called an infrared spectrometer which produces an infrared spectrum. An IR spectrum can be visualized in a graph of infrared light absorbance on the vertical axis vs. frequency or wavelength on the horizontal axis. Typical units of frequency used in IR spectra are reciprocal centimeters, with the symbol cm−1. Units of IR wavelength are commonly given in micrometers, symbol μm, which are related to wave numbers in a reciprocal way. A common laboratory instrument that uses this technique is a Fourier transform infrared (FTIR) spectrometer. Two-dimensional IR is also possible as discussed below.

Organic chemistry is a branch of chemistry that studies the structure, properties and reactions of organic compounds, which contain carbon-carbon covalent bonds. Study of structure determines their structural formula. Study of properties includes physical and chemical properties, and evaluation of chemical reactivity to understand their behavior. The study of organic reactions includes the chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical study.

Spectroscopy is the general field of study that measures and interprets the electromagnetic spectra that result from the interaction between electromagnetic radiation and matter as a function of the wavelength or frequency of the radiation. Matter waves and acoustic waves can also be considered forms of radiative energy, and recently gravitational waves have been associated with a spectral signature in the context of the Laser Interferometer Gravitational-Wave Observatory (LIGO)
Chemometrics is the science of extracting information from chemical systems by data-driven means. Chemometrics is inherently interdisciplinary, using methods frequently employed in core data-analytic disciplines such as multivariate statistics, applied mathematics, and computer science, in order to address problems in chemistry, biochemistry, medicine, biology and chemical engineering. In this way, it mirrors other interdisciplinary fields, such as psychometrics and econometrics.

Spectrophotometry is a branch of electromagnetic spectroscopy concerned with the quantitative measurement of the reflection or transmission properties of a material as a function of wavelength. Spectrophotometry uses photometers, known as spectrophotometers, that can measure the intensity of a light beam at different wavelengths. Although spectrophotometry is most commonly applied to ultraviolet, visible, and infrared radiation, modern spectrophotometers can interrogate wide swaths of the electromagnetic spectrum, including x-ray, ultraviolet, visible, infrared, and/or microwave wavelengths.

The post-mortem interval (PMI) is the time that has elapsed since an individual's death. When the time of death is not known, the interval may be estimated, and so an approximate time of death established. There are standard medical and scientific techniques supporting such an estimation.

Laser-induced breakdown spectroscopy (LIBS) is a type of atomic emission spectroscopy which uses a highly energetic laser pulse as the excitation source. The laser is focused to form a plasma, which atomizes and excites samples. The formation of the plasma only begins when the focused laser achieves a certain threshold for optical breakdown, which generally depends on the environment and the target material.
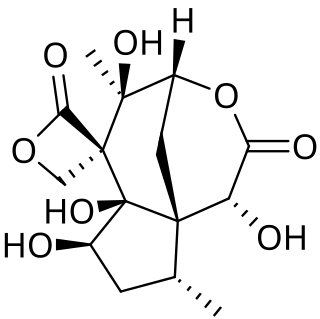
Forensic chemistry is the application of chemistry and its subfield, forensic toxicology, in a legal setting. A forensic chemist can assist in the identification of unknown materials found at a crime scene. Specialists in this field have a wide array of methods and instruments to help identify unknown substances. These include high-performance liquid chromatography, gas chromatography-mass spectrometry, atomic absorption spectroscopy, Fourier transform infrared spectroscopy, and thin layer chromatography. The range of different methods is important due to the destructive nature of some instruments and the number of possible unknown substances that can be found at a scene. Forensic chemists prefer using nondestructive methods first, to preserve evidence and to determine which destructive methods will produce the best results.
Characterization, when used in materials science, refers to the broad and general process by which a material's structure and properties are probed and measured. It is a fundamental process in the field of materials science, without which no scientific understanding of engineering materials could be ascertained. The scope of the term often differs; some definitions limit the term's use to techniques which study the microscopic structure and properties of materials, while others use the term to refer to any materials analysis process including macroscopic techniques such as mechanical testing, thermal analysis and density calculation. The scale of the structures observed in materials characterization ranges from angstroms, such as in the imaging of individual atoms and chemical bonds, up to centimeters, such as in the imaging of coarse grain structures in metals.
An infrared spectroscopy correlation table is a list of absorption peaks and frequencies, typically reported in wavenumber, for common types of molecular bonds and functional groups. In physical and analytical chemistry, infrared spectroscopy is a technique used to identify chemical compounds based on the way infrared radiation is absorbed by the compound.
Applied spectroscopy is the application of various spectroscopic methods for the detection and identification of different elements or compounds to solve problems in fields like forensics, medicine, the oil industry, atmospheric chemistry, and pharmacology.
Chemical imaging is the analytical capability to create a visual image of components distribution from simultaneous measurement of spectra and spatial, time information. Hyperspectral imaging measures contiguous spectral bands, as opposed to multispectral imaging which measures spaced spectral bands.

Instrumental analysis is a field of analytical chemistry that investigates analytes using scientific instruments.
Applied Spectroscopy is a peer-reviewed scientific journal published monthly by the Society for Applied Spectroscopy, and it is also the official journal for this society. The editor-in-chief is Sergei G. Kazarian. The journal covers applications of spectroscopy in analytical chemistry, materials science, biotechnology, and chemical characterization.
Deformulation refers to a set of analytical procedures used to separate and identify individual components of a formulated chemical substance. Deformulation applies methods of analytical chemistry and is often used to obtain competitive intelligence about chemical products. Deformulation is related to reverse engineering; however, the latter concept is most closely associated with procedures used to discover working principles of a device or a designed system through examination and disassembly of its structure. The term, reverse engineering, has become specifically and almost exclusively linked to the field of software engineering; whereas, deformulation is a term more applicable to the field of chemical manufacturing. Deformulation of a multicomponent chemical mixture may occur in several contexts, including the investigation of causes of chemical product failure, competitive benchmarking, legal inquiry to obtain evidence of patent infringement, or new product research and development. Depending upon this context and upon the level of information sought, the requirements of analyses for deformulation may differ. Deformulation processes typically require the application of several analytical methods, and the selection of methods is dependent upon the degree of confidence required in the results. Methods of deformulation also have similarity to methods of forensic chemistry in which analytical procedures may be applied to discover the causes of material failure or to resolve a legal question.
Yukihiro Ozaki is a Japanese scientist. Kwansei Gakuin University, Department of Chemistry, School of Science and Technology, professor emeritus, Fellow.
R. Robert Brattain was an American physicist at Shell Development Company. He was involved in a number of secret projects during World War II. He is recognized as one of America's leading infrared spectroscopists for his work in designing several models of spectrophotometer, and for using the infrared spectrophotometer to determine the β-lactam structure of penicillin. His instrumentation work was essential to the subsequent study and understanding of structures in organic chemistry.

Reiner Salzer is a German chemist and university teacher of Analytical Chemistry at the TU Dresden.
Mitsuo Tasumi was a Japanese physical chemist known for his vibrational spectroscopic works on synthetic and biological macromolecules. He was Professor Emeritus of the University of Tokyo, and a former president of Saitama University, having trained a number of physical chemists active in academia and industry. Moto-o Tasumi, a zoologist at Kyoto University, was his brother.
This article needs additional or more specific categories .(January 2022) |