In polymer chemistry, polymerization, or polymerisation, is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many forms of polymerization and different systems exist to categorize them.

Petrochemicals are the chemical products obtained from petroleum by refining. Some chemical compounds made from petroleum are also obtained from other fossil fuels, such as coal or natural gas, or renewable sources such as maize, palm fruit or sugar cane.
In polymer chemistry, ring-opening polymerization (ROP) is a form of chain-growth polymerization, in which the terminus of a polymer chain attacks cyclic monomers to form a longer polymer. The reactive center can be radical, anionic or cationic. Some cyclic monomers such as norbornene or cyclooctadiene can be polymerized to high molecular weight polymers by using metal catalysts. ROP is a versatile method for the synthesis of biopolymers.

A thermosetting polymer, resin, or plastic, often called a thermoset, is a polymer that is irreversibly hardened by curing from a soft solid or viscous liquid prepolymer or resin. Curing is induced by heat or suitable radiation and may be promoted by high pressure, or mixing with a catalyst. Heat is not necessarily to be applied externally. It is often generated by the reaction of the resin with a curing agent. Curing results in chemical reactions that create extensive cross-linking between polymer chains to produce an infusible and insoluble polymer network.

Isosorbide is a bicyclic chemical compound from the group of diols and the oxygen-containing heterocycles, containing two fused furan rings. The starting material for isosorbide is D-sorbitol, which is obtained by catalytic hydrogenation of D-glucose, which is in turn produced by hydrolysis of starch. Isosorbide is discussed as a plant-based platform chemical from which biodegradable derivatives of various functionality can be obtained.

Orthocarbonic acid (methanetetrol) is the name given to a hypothetical compound with the chemical formula H4CO4 or C(OH)4. Its molecular structure consists of a single carbon atom bonded to four hydroxy groups. It would be therefore a fourfold alcohol. In theory it could lose four protons to give the hypothetical oxocarbon anion CO4−
4 (orthocarbonate), and is therefore considered an oxoacid of carbon.
Coordination polymerisation is a form of polymerization that is catalyzed by transition metal salts and complexes.

Polyester is a category of polymers that contain the ester functional group in every repeat unit of their main chain. As a specific material, it most commonly refers to a type called polyethylene terephthalate (PET). Polyesters include naturally occurring chemicals, such as in the cutin of plant cuticles, as well as synthetics such as polybutyrate. Natural polyesters and a few synthetic ones are biodegradable, but most synthetic polyesters are not. The material is used extensively in clothing.
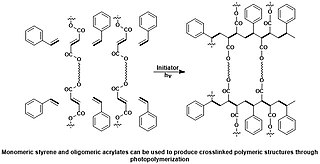
A photopolymer or light-activated resin is a polymer that changes its properties when exposed to light, often in the ultraviolet or visible region of the electromagnetic spectrum. These changes are often manifested structurally, for example hardening of the material occurs as a result of cross-linking when exposed to light. An example is shown below depicting a mixture of monomers, oligomers, and photoinitiators that conform into a hardened polymeric material through a process called curing.
A glucan is a polysaccharide derived from D-glucose, linked by glycosidic bonds. Many beta-glucans are medically important. They represent a drug target for antifungal medications of the echinocandin class.
Synthetic resins are industrially produced resins, typically viscous substances that convert into rigid polymers by the process of curing. In order to undergo curing, resins typically contain reactive end groups, such as acrylates or epoxides. Some synthetic resins have properties similar to natural plant resins, but many do not.

Oxazines are heterocyclic compounds containing one oxygen and one nitrogen atom in a doubly unsaturated six-membered ring. Isomers exist depending on the relative position of the heteroatoms and relative position of the double bonds.
Polybenzoxazines, also called benzoxazine resins, are cured polymerization products derived from benzoxazine monomers.
Chain transfer is a polymerization reaction by which the activity of a growing polymer chain is transferred to another molecule.
A thermoset polymer matrix is a synthetic polymer reinforcement where polymers act as binder or matrix to secure in place incorporated particulates, fibres or other reinforcements. They were first developed for structural applications, such as glass-reinforced plastic radar domes on aircraft and graphite-epoxy payload bay doors on the space shuttle.

Oxazoline is a five-membered heterocyclic chemical compound containing one atom each of oxygen and nitrogen. It was likely first synthesized in 1884 but it was not until 5 years later that Siegmund Gabriel correctly assigned the structure. It was named in-line with the Hantzsch–Widman nomenclature and is part of a family of heterocyclic compounds, where it exists between oxazole and oxazolidine in terms of saturation.

Levopimaric acid is an abietane-type of diterpene resin acid. It is a major constituent of pine oleoresin with the chemical formula of C20H30O2. In general, the abietene types of diterpene resin acid have various biological activities, such as antibacterial, cardiovascular and antioxidant. About 18 to 25% of levopimaric acid is found in pine oleoresin. The production of oleoresin by conifer species is an important component of the defense response against insect attack and fungal pathogen infection.
IUPAC Polymer Nomenclature are standardized naming conventions for polymers set by the International Union of Pure and Applied Chemistry (IUPAC) and described in their publication "Compendium of Polymer Terminology and Nomenclature", which is also known as the "Purple Book". Both the IUPAC and Chemical Abstracts Service (CAS) make similar naming recommendations for the naming of polymers.
Nylon 12 is a polymer with the formula [(CH2)11C(O)NH]n. It is made from ω-aminolauric acid or laurolactam monomers that each have 12 carbons, hence the name ‘Nylon 12’. It is one of several nylon polymers.
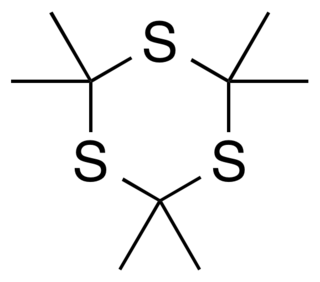
Trithioacetone (2,2,4,4,6,6-hexamethyl-1,3,5-trithiane) is an organic chemical with formula C
9H
18S
3. Its covalent structure is [–C(CH
3)
2–S–]
3, that is, a six-membered ring of alternating carbon and sulfur atoms, with two methyl groups attached to each carbon. It can be viewed as a derivative of 1,3,5-trithiane, with methyl-group substituents for all of the hydrogen atoms in that parent structure.










