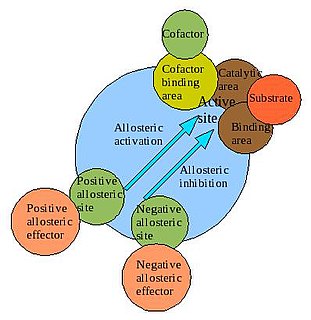
In biochemistry, allosteric regulation is the regulation of an enzyme by binding an effector molecule at a site other than the enzyme's active site.

Drug design, often referred to as rational drug design or simply rational design, is the inventive process of finding new medications based on the knowledge of a biological target. The drug is most commonly an organic small molecule that activates or inhibits the function of a biomolecule such as a protein, which in turn results in a therapeutic benefit to the patient. In the most basic sense, drug design involves the design of molecules that are complementary in shape and charge to the biomolecular target with which they interact and therefore will bind to it. Drug design frequently but not necessarily relies on computer modeling techniques. This type of modeling is sometimes referred to as computer-aided drug design. Finally, drug design that relies on the knowledge of the three-dimensional structure of the biomolecular target is known as structure-based drug design. In addition to small molecules, biopharmaceuticals including peptides and especially therapeutic antibodies are an increasingly important class of drugs and computational methods for improving the affinity, selectivity, and stability of these protein-based therapeutics have also been developed.
The term molecular recognition refers to the specific interaction between two or more molecules through noncovalent bonding such as hydrogen bonding, metal coordination, hydrophobic forces, van der Waals forces, π-π interactions, halogen bonding, electrostatic and/or electromagnetic effects. In addition to these direct interactions as well solvent can play a dominant indirect role in driving molecular recognition in solution. The host and guest involved in molecular recognition exhibit molecular complementarity.Exceptions are molecular containers, including e.g. nanotubes, in which portals essentially control selectivity.
Aptamers are oligonucleotide or peptide molecules that bind to a specific target molecule. Aptamers are usually created by selecting them from a large random sequence pool, but natural aptamers also exist in riboswitches. Aptamers can be used for both basic research and clinical purposes as macromolecular drugs. Aptamers can be combined with ribozymes to self-cleave in the presence of their target molecule. These compound molecules have additional research, industrial and clinical applications.

Protein–protein interactions (PPIs) are the physical contacts of high specificity established between two or more protein molecules as a result of biochemical events steered by electrostatic forces including the hydrophobic effect. Many are physical contacts with molecular associations between chains that occur in a cell or in a living organism in a specific biomolecular context.

In biochemistry and pharmacology, a ligand is a substance that forms a complex with a biomolecule to serve a biological purpose. In protein-ligand binding, the ligand is usually a molecule which produces a signal by binding to a site on a target protein. The binding typically results in a change of conformational isomerism (conformation) of the target protein. In DNA-ligand binding studies, the ligand can be a small molecule, ion, or protein which binds to the DNA double helix. The relationship between ligand and binding partner is a function of charge, hydrophobicity, and molecular structure. The instance of binding occurs over an infinitesimal range of time and space, so the rate constant is usually a very small number.
The DrugBank database is a comprehensive, freely accessible, online database containing information on drugs and drug targets. As both a bioinformatics and a cheminformatics resource, DrugBank combines detailed drug data with comprehensive drug target information. DrugBank uses a fair bit of content from Wikipedia. Wikipedia also often links to Drugbank.
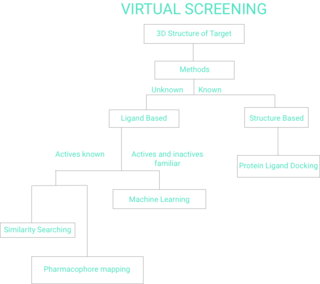
Virtual screening (VS) is a computational technique used in drug discovery to search libraries of small molecules in order to identify those structures which are most likely to bind to a drug target, typically a protein receptor or enzyme.
In the fields of computational chemistry and molecular modelling, scoring functions are mathematical functions used to approximately predict the binding affinity between two molecules after they have been docked. Most commonly one of the molecules is a small organic compound such as a drug and the second is the drug's biological target such as a protein receptor. Scoring functions have also been developed to predict the strength of intermolecular interactions between two proteins or between protein and DNA.
The PDBbind database is a comprehensive collection of experimentally measured binding affinity data for the protein-ligand complexes deposited in the Protein Data Bank (PDB). It thus provides a link between energetic and structural information of protein-ligand complexes, which is of great value to various studies on molecular recognition occurred in biological systems.
Fragment-based lead discovery (FBLD) also known as fragment-based drug discovery (FBDD) is a method used for finding lead compounds as part of the drug discovery process. Fragments are small organic molecules which are small in size and low in molecular weight. It is based on identifying small chemical fragments, which may bind only weakly to the biological target, and then growing them or combining them to produce a lead with a higher affinity. FBLD can be compared with high-throughput screening (HTS). In HTS, libraries with up to millions of compounds, with molecular weights of around 500 Da, are screened, and nanomolar binding affinities are sought. In contrast, in the early phase of FBLD, libraries with a few thousand compounds with molecular weights of around 200 Da may be screened, and millimolar affinities can be considered useful. FBLD is a technique being used in research for discovering novel potent inhibitors.

Affinity electrophoresis is a general name for many analytical methods used in biochemistry and biotechnology. Both qualitative and quantitative information may be obtained through affinity electrophoresis. The methods include the so-called electrophoretic mobility shift assay, charge shift electrophoresis and affinity capillary electrophoresis. The methods are based on changes in the electrophoretic pattern of molecules through biospecific interaction or complex formation. The interaction or binding of a molecule, charged or uncharged, will normally change the electrophoretic properties of a molecule. Membrane proteins may be identified by a shift in mobility induced by a charged detergent. Nucleic acids or nucleic acid fragments may be characterized by their affinity to other molecules. The methods have been used for estimation of binding constants, as for instance in lectin affinity electrophoresis or characterization of molecules with specific features like glycan content or ligand binding. For enzymes and other ligand-binding proteins, one-dimensional electrophoresis similar to counter electrophoresis or to "rocket immunoelectrophoresis", affinity electrophoresis may be used as an alternative quantification of the protein. Some of the methods are similar to affinity chromatography by use of immobilized ligands.
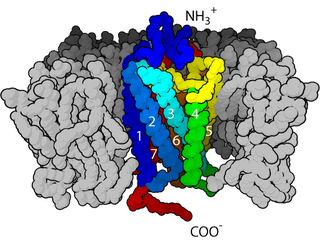
Cell surface receptors are receptors that are embedded in the plasma membrane of cells. They act in cell signaling by receiving extracellular molecules. They are specialized integral membrane proteins that allow communication between the cell and the extracellular space. The extracellular molecules may be hormones, neurotransmitters, cytokines, growth factors, cell adhesion molecules, or nutrients; they react with the receptor to induce changes in the metabolism and activity of a cell. In the process of signal transduction, ligand binding affects a cascading chemical change through the cell membrane.

In molecular biology Short Linear Motifs are short stretches of protein sequence that mediate protein–protein interaction.
Computational Resources for Drug Discovery (CRDD) is one of the important silico modules of Open Source for Drug Discovery (OSDD). The CRDD web portal provides computer resources related to drug discovery on a single platform. It provides computational resources for researchers in computer-aided drug design, a discussion forum, and resources to maintain Wikipedia related to drug discovery, predict inhibitors, and predict the ADME-Tox property of molecules One of the major objectives of CRDD is to promote open source software in the field of chemoinformatics and pharmacoinformatics.
Allostery is the most direct and efficient way for regulation of biological macromolecule function induced by the binding of a ligand at an allosteric site topographically distinct from the orthosteric site. Due to the inherent high receptor selectivity and lower target-based toxicity, it is also expected to play a more positive role in drug discovery and bioengineering, leading to rapid growth on allosteric findings.
A thermal shift assay quantifies the change in thermal denaturation temperature of a protein under varying conditions. The differing conditions that can be examined are very diverse, e.g. pH, salts, additives, drugs, drug leads, oxidation/reduction, or mutations. The binding of low molecular weight ligands can increase the thermal stability of a protein, as described by Daniel Koshland (1958) and Kaj Ulrik Linderstrøm-Lang and Schellman (1959). Almost half of enzymes require a metal ion co-factor. Thermostable proteins are often more useful than their non-thermostable counterparts, e.g. DNA polymerase in the polymerase chain reaction, so protein engineering often includes adding mutations to increase thermal stability. Protein crystallisation is more successful for proteins with a higher melting point and adding buffer components that stabilise proteins improve the likelihood of protein crystals forming. If examining pH then the possible effects of the buffer molecule on thermal stability should be taken into account along with the fact that pKa of each buffer molecule changes uniquely with temperature. Additionally, any time a charged species is examined the effects of the counterion should be accounted for.
Ligand binding assays (LBA) is an assay, or an analytic procedure, whose procedure or method relies on the binding of ligand molecules to receptors, antibodies or other macromolecules. A detection method is used to determine the presence and extent of the ligand-receptor complexes formed, and this is usually determined electrochemically or through a fluorescence detection method. This type of analytic test can be used to test for the presence of target molecules in a sample that are known to bind to the receptor.
SAMPL is a set of community-wide blind challenges aimed to advance computational techniques as standard predictive tools in rational drug design. A broad range of biologically relevant systems with different sizes and levels of complexities including proteins, host–guest complexes, and drug-like small molecules have been selected to test the latest modeling methods and force fields in SAMPL. New experimental data, such as binding affinity and hydration free energy, are withheld from participants until the prediction submission deadline, so that the true predictive power of methods can be revealed. The most recent SAMPL5 challenge contains two prediction categories: the binding affinity of host–guest systems, and the distribution coefficients of drug-like molecules between water and cyclohexane. Since 2008, the SAMPL challenge series has attracted widespread interest from scientists engaged in the field of computer-aided drug design (CADD) around the world, and has resulted in well over 100 publications with many of them highly cited. The current SAMPL organizers include Prof. John Chodera at Memorial Sloan Kettering Cancer Center, Prof. Michael K. Gilson at University of California, San Diego, Prof. David Mobley at University of California, Irvine, and Prof. Michael Shirts, at University of Colorado, Boulder.

![Host–guest chemistry [[Supramolecular chemistry|Supramolecular structures]] held together other than by covalent bonds](https://upload.wikimedia.org/wikipedia/commons/thumb/9/98/Cucurbit-6-uril_ActaCrystallB-Stru_1984_382.jpg/320px-Cucurbit-6-uril_ActaCrystallB-Stru_1984_382.jpg)













