SNARE proteins – "SNApREceptor" – are a large protein family consisting of at least 24 members in yeasts, more than 60 members in mammalian cells, and some numbers in plants. The primary role of SNARE proteins is to mediate vesicle fusion – the fusion of vesicles with the target membrane; this notably mediates exocytosis, but can also mediate the fusion of vesicles with membrane-bound compartments. The best studied SNAREs are those that mediate the release of synaptic vesicles containing neurotransmitter in neurons. These neuronal SNAREs are the targets of the neurotoxins responsible for botulism and tetanus produced by certain bacteria.

Synaptosomal-Associated Protein, 25kDa (SNAP-25) is a Target Soluble NSF (N-ethylmaleimide-sensitive factor) Attachment Protein Receptor (t-SNARE) protein encoded by the SNAP25 gene found on chromosome 20p12.2 in humans. SNAP-25 is a component of the trans-SNARE complex, which accounts for membrane fusion specificity and directly executes fusion by forming a tight complex that brings the synaptic vesicle and plasma membranes together.

Syntaxin-1A is a protein that in humans is encoded by the STX1A gene.

Synaptosomal-associated protein 23 is a protein that in humans is encoded by the SNAP23 gene. Two alternative transcript variants encoding different protein isoforms have been described for this gene.

Syntaxin-4 is a protein that in humans is encoded by the STX4 gene.

Vesicle-associated membrane protein 2 (VAMP2) is a protein that in humans is encoded by the VAMP2 gene.

Vesicle-associated membrane protein 7 (VAMP-7), is a protein that in humans is encoded by the VAMP7 gene also known as the or SYBL1 gene.

Syntaxin-6 is a protein that in humans is encoded by the STX6 gene.

N-ethylmaleimide-sensitive factor Attachment Protein Alpha, also known as SNAP-α, is a SNAP protein that is involved in the intra-cellular trafficking and fusing of vesicles to target membranes in cells.

Vesicle-associated membrane protein 3 is a protein that in humans is encoded by the VAMP3 gene.

Syntaxin-5 is a protein that in humans is encoded by the STX5 gene.

Synaptobrevin homolog YKT6 is a protein that in humans is encoded by the YKT6 gene.

Golgi SNAP receptor complex member 1 is a protein that in humans is encoded by the GOSR1 gene.

Gamma-soluble NSF attachment protein is a SNAP protein that in humans is encoded by the NAPG gene.

Golgi SNAP receptor complex member 2 is a protein that in humans is encoded by the GOSR2 gene.

Beta-soluble NSF attachment protein is a SNAP protein involved in vesicular trafficking and exocytosis which is encoded by the NAPB gene humans is.

Autophagy-related protein 8 (Atg8) is a ubiquitin-like protein required for the formation of autophagosomal membranes. The transient conjugation of Atg8 to the autophagosomal membrane through a ubiquitin-like conjugation system is essential for autophagy in eukaryotes. Even though there are homologues in animals, this article mainly focuses on its role in lower eukaryotes such as Saccharomyces cerevisiae.

Syntaxin-10 (STX10) is a SNARE protein that is encoded by the STX10 gene. This protein is found in most vertebrates but is noticeably absent from mice. As with other SNARE proteins, STX10 facilitates vesicle fusion and thus is important for intracellular trafficking of proteins and other cellular components. More specifically, STX10 has been implicated in endosome to Golgi trafficking of the mannose 6-phosphate receptor and glucose transporter type 4.

Soluble N-ethylmaleimide-Sensitive Factor Attachment Proteins are a family of cytosolic adaptor proteins involved in vesicular fusion at membranes during intracellular transport and exocytosis. SNAPs interact with proteins of the SNARE complex and NSF to play a key role in recycling the components of the fusion complex. SNAPs are involved in the priming of the vesicle fusion complex during assembly, as well as in the disassembly following a vesicle fusion event. Following membrane fusion, the tethering SNARE proteins complex disassembles in response to steric changes originating from the ATPase NSF. The energy provided by NSF is transferred throughout the SNARE complex and SNAP, allowing the proteins to untangle, and recycled for future fusion events. Mammals have three SNAP genes: α-SNAP, β-SNAP, and γ-SNAP. α- and γ-SNAP are expressed throughout the body, while β-SNAP is specific to the brain. The yeast homolog of the human SNAP is Sec17, the structural diagram of which is included on this page.
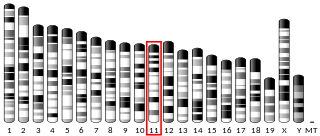
Synaptosome-associated protein, 47 kDal (SNAP47) is a human protein encoded by the SNAP47 gene. Other aliases of this gene are SVAP1, HEL170, ESFI5812, and HEL-S-290. SNAP47 is a synaptosome protein which is associated with the protein coding in multiple diseases, including non small cell lung cancer and schizophrenia. SNAP47 is a member of the SNAP protein family. SNAP proteins are t-snare proteins that are a component of SNARE complex. The SNARE complex mediates vesicle fusion by creating tight complex that brings vesicle and membrane together. This protein causes ubiquitous expression in testis, ovary, and many other tissues