Related Research Articles

Hemoglobin is a protein containing iron that facilitates the transportation of oxygen in red blood cells. Almost all vertebrates contain hemoglobin, with the sole exception of the fish family Channichthyidae. Hemoglobin in the blood carries oxygen from the respiratory organs to the other tissues of the body, where it releases the oxygen to enable aerobic respiration which powers an animal's metabolism. A healthy human has 12 to 20 grams of hemoglobin in every 100 mL of blood. Hemoglobin is a metalloprotein, a chromoprotein, and globulin.

Functional magnetic resonance imaging or functional MRI (fMRI) measures brain activity by detecting changes associated with blood flow. This technique relies on the fact that cerebral blood flow and neuronal activation are coupled. When an area of the brain is in use, blood flow to that region also increases.

In haemodynamics, the body must respond to physical activities, external temperature, and other factors by homeostatically adjusting its blood flow to deliver nutrients such as oxygen and glucose to stressed tissues and allow them to function. Haemodynamic response (HR) allows the rapid delivery of blood to active neuronal tissues. The brain consumes large amounts of energy but does not have a reservoir of stored energy substrates. Since higher processes in the brain occur almost constantly, cerebral blood flow is essential for the maintenance of neurons, astrocytes, and other cells of the brain. This coupling between neuronal activity and blood flow is also referred to as neurovascular coupling.

Magnetic resonance angiography (MRA) is a group of techniques based on magnetic resonance imaging (MRI) to image blood vessels. Magnetic resonance angiography is used to generate images of arteries in order to evaluate them for stenosis, occlusions, aneurysms or other abnormalities. MRA is often used to evaluate the arteries of the neck and brain, the thoracic and abdominal aorta, the renal arteries, and the legs.
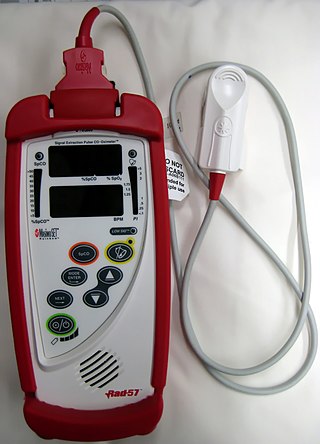
A CO-oximeter is a device that measures the oxygen carrying state of hemoglobin in a blood specimen, including oxygen-carrying hemoglobin (O2Hb), non-oxygen-carrying but normal hemoglobin (HHb), as well as the dyshemoglobins such as carboxyhemoglobin (COHb) and methemoglobin (MetHb). The use of 'CO' rather than 'Co' or 'co' is more appropriate since this designation represents a device that measures carbon monoxide (CO) bound to hemoglobin, as distinguished from simple oximetry which measures hemoglobin bound to molecular oxygen—O2Hb—or hemoglobin capable of binding to molecular oxygen—HHb. Simpler oximeters may report oxygen saturation alone, i.e. the ratio of oxyhemoglobin to total 'bindable' hemoglobin. CO-oximetry is useful in defining the causes for hypoxemia, or hypoxia,.

Neuroimaging is the use of quantitative (computational) techniques to study the structure and function of the central nervous system, developed as an objective way of scientifically studying the healthy human brain in a non-invasive manner. Increasingly it is also being used for quantitative research studies of brain disease and psychiatric illness. Neuroimaging is highly multidisciplinary involving neuroscience, computer science, psychology and statistics, and is not a medical specialty. Neuroimaging is sometimes confused with neuroradiology.
The Haldane effect is a property of hemoglobin first described by John Scott Haldane, within which oxygenation of blood in the lungs displaces carbon dioxide from hemoglobin, increasing the removal of carbon dioxide. Consequently, oxygenated blood has a reduced affinity for carbon dioxide. Thus, the Haldane effect describes the ability of hemoglobin to carry increased amounts of carbon dioxide (CO2) in the deoxygenated state as opposed to the oxygenated state. Vice versa, it is true that a high concentration of CO2 facilitates dissociation of oxyhemoglobin, though this is the result of two distinct processes (Bohr effect and Margaria-Green effect) and should be distinguished from Haldane effect.

Seiji Ogawa is a Japanese biophysicist and neuroscientist known for discovering the technique that underlies Functional Magnetic Resonance Imaging (fMRI). He is regarded as the father of modern functional brain imaging. He determined that the changes in blood oxygen levels cause its magnetic resonance imaging properties to change, allowing a map of blood, and hence, functional, activity in the brain to be created. This map reflected which neurons of the brain responded with electrochemical signals to mental processes. He was the first scientist who demonstrated that the functional brain imaging is dependent on the oxygenation status of the blood, the BOLD effect. The technique was therefore called blood oxygenation level-dependent or BOLD contrast. Functional MRI (fMRI) has been used to map the visual, auditory, and sensory regions and moving toward higher brain functions such as cognitive functions in the brain.
Kenneth Kin Man Kwong is a Hong Kong-born American nuclear physicist. He is a pioneer in human brain imaging. He received his bachelor's degree in Political Science in 1972 from the University of California, Berkeley. He went on to receive his Ph.D. in physics from the University of California, Riverside studying photon-photon collision interactions.
Event-related functional magnetic resonance imaging (efMRI) is a technique used in magnetic resonance imaging of medical patients.
Neurophysics is the branch of biophysics dealing with the development and use of physical methods to gain information about the nervous system. Neurophysics is an interdisciplinary science using physics and combining it with other neurosciences to better understand neural processes. The methods used include the techniques of experimental biophysics and other physical measurements such as EEG mostly to study electrical, mechanical or fluidic properties, as well as theoretical and computational approaches. The term "neurophysics" is a portmanteau of "neuron" and "physics".
Signal enhancement by extravascular water protons, or SEEP, is a contrast mechanism for functional magnetic resonance imaging (fMRI), which is an alternative to the more commonly employed BOLD contrast. This mechanism for image contrast changes corresponding to changes in neuronal activity was first proposed by Dr. Patrick Stroman in 2001. SEEP contrast is based on changes in tissue water content which arise from the increased production of extracellular fluid and swelling of neurons and glial cells at sites of neuronal activity. Because the dominant sources of MRI signal in biological tissues are water and lipids, an increase in tissue water content is reflected by a local increase in MR signal intensity. A correspondence between BOLD and SEEP signal changes, and sites of activity, has been observed in the brain and appears to arise from the common dependence on changes in local blood flow to cause a change in blood oxygenation or to produce extracellular fluid. The advantage of SEEP contrast is that it can be detected with MR imaging methods which are relatively insensitive to magnetic susceptibility differences between air, tissues, blood, and bone. Such susceptibility differences can give rise to spatial image distortions and areas of low signal, and magnetic susceptibility changes in blood give rise to the BOLD contrast for fMRI. The primary application of SEEP to date has been fMRI of the spinal cord because the bone/tissue interfaces around the spinal cord cause poor image quality with conventional fMRI methods. The disadvantages of SEEP compared to BOLD contrast are that it reveals more localized areas of activity, and in the brain the signal intensity changes are typically lower, and it can therefore be more difficult to detect.
Functional magnetic resonance imaging (fMRI) of the spinal cord is an adaptation of the fMRI method that has been developed for use in the brain. Although the basic principles underlying the methods are the same, spinal fMRI requires a number of specific adaptations to accommodate the periodic motion of the spinal cord, the small cross-sectional dimensions and length of the spinal cord, and the fact that the magnetic field that is used for MRI varies with position in the spinal cord because of magnetic susceptibility differences between bone and tissues. Spinal fMRI has been used to produce maps of neuronal activity at most levels of the spinal cord in response to various stimuli, such as touch, vibration, and thermal changes, and with motor tasks. Research applications of spinal fMRI to date include studies of normal sensory and motor function, and studies of the effects of trauma and multiple sclerosis on the spinal cord.
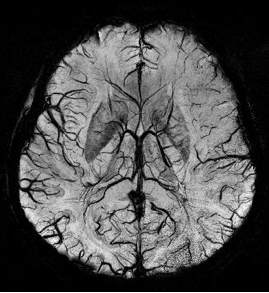
Susceptibility weighted imaging (SWI), originally called BOLD venographic imaging, is an MRI sequence that is exquisitely sensitive to venous blood, hemorrhage and iron storage. SWI uses a fully flow compensated, long echo, gradient recalled echo (GRE) pulse sequence to acquire images. This method exploits the susceptibility differences between tissues and uses the phase image to detect these differences. The magnitude and phase data are combined to produce an enhanced contrast magnitude image. The imaging of venous blood with SWI is a blood-oxygen-level dependent (BOLD) technique which is why it was referred to as BOLD venography. Due to its sensitivity to venous blood SWI is commonly used in traumatic brain injuries (TBI) and for high resolution brain venographies but has many other clinical applications. SWI is offered as a clinical package by Philips and Siemens but can be run on any manufacturer's machine at field strengths of 1.0 T, 1.5 T, 3.0 T and higher.

Magnetic resonance imaging of the brain uses magnetic resonance imaging (MRI) to produce high-quality two- or three-dimensional images of the brain, brainstem, and cerebellum without ionizing radiation (X-rays) or radioactive tracers.
The temporal dynamics of music and language describes how the brain coordinates its different regions to process musical and vocal sounds. Both music and language feature rhythmic and melodic structure. Both employ a finite set of basic elements that are combined in ordered ways to create complete musical or lingual ideas.

Resting state fMRI, also referred to as task-independent fMRI or task-free fMRI, is a method of functional magnetic resonance imaging (fMRI) that is used in brain mapping to evaluate regional interactions that occur in a resting or task-negative state, when an explicit task is not being performed. A number of resting-state brain networks have been identified, one of which is the default mode network. These brain networks are observed through changes in blood flow in the brain which creates what is referred to as a blood-oxygen-level dependent (BOLD) signal that can be measured using fMRI.
Functional magnetic resonance spectroscopy of the brain (fMRS) uses magnetic resonance imaging (MRI) to study brain metabolism during brain activation. The data generated by fMRS usually shows spectra of resonances, instead of a brain image, as with MRI. The area under peaks in the spectrum represents relative concentrations of metabolites.
The following outline is provided as an overview of and topical guide to brain mapping:

An MRI pulse sequence in magnetic resonance imaging (MRI) is a particular setting of pulse sequences and pulsed field gradients, resulting in a particular image appearance.
References
- 1 2 E. Raichle, Marcus (2010). "The Brain's Dark Energy". Scientific American. 302 (3): 44–49. Bibcode:2010SciAm.302c..44R. doi:10.1038/scientificamerican0310-44. PMID 20184182.
The fMRI signal is usually referred to as the blood-oxygen-level-dependent (BOLD) signal because the imaging method relies on changes in the level of oxygen in the human brain induced by alterations in blood flow.
- ↑ Ogawa, S.; Lee, T. M.; Kay, A. R.; Tank, D. W. (1990). "Brain magnetic resonance imaging with contrast dependent on blood oxygenation". Proceedings of the National Academy of Sciences. 87 (24): 9868–9872. Bibcode:1990PNAS...87.9868O. doi: 10.1073/pnas.87.24.9868 . PMC 55275 . PMID 2124706.
- ↑ Chou, I-han. "Milestone 19: (1990) Functional MRI". Nature. Retrieved 9 August 2013.
- ↑ Yablonskiy, Dmitriy A.; Haacke, E. Mark (1994). "Theory of NMR signal behavior in magnetically inhomogeneous tissues: The static dephasing regime". Magnetic Resonance in Medicine. 32 (6): 749–763. doi:10.1002/mrm.1910320610. PMID 7869897.
- ↑ Langleben, Daniel D. (1 February 2008). "Detection of deception with fMRI: Are we there yet?". Legal and Criminological Psychology. 13 (1): 1–9. doi:10.1348/135532507X251641.
- ↑ Raichle, ME (3 February 1998). "Behind the scenes of functional brain imaging: a historical and physiological perspective". Proceedings of the National Academy of Sciences of the United States of America. 95 (3): 765–72. Bibcode:1998PNAS...95..765R. doi: 10.1073/pnas.95.3.765 . PMC 33796 . PMID 9448239.
Ogawa et al. were able to demonstrate that in vivo changes blood oxygenation could be detected with MRI.
- ↑ OGAWA, SEIJI (1990). "Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields". Magnetic Resonance in Medicine. 14 (1): 68–78. doi:10.1002/mrm.1910140108. PMID 2161986. S2CID 12379024.
- ↑ Roche, Richard A.P.; Commins, Seán; Dockree, Paul M. (2009). "Cognitive neuroscience: introduction and historical perspective". In Roche, Richard A.P.; Commins, Seán (eds.). Pioneering studies in cognitive neuroscience. Maidenhead, Berkshire: McGraw Hill Open University Press. p. 11. ISBN 978-0335233564.