Related Research Articles

Gastrin is a peptide hormone that stimulates secretion of gastric acid (HCl) by the parietal cells of the stomach and aids in gastric motility. It is released by G cells in the pyloric antrum of the stomach, duodenum, and the pancreas.
Bombesin is a 14-amino acid peptide originally isolated from the skin of the European fire-bellied toad. It has two known homologs in mammals called neuromedin B and gastrin-releasing peptide. It stimulates gastrin release from G cells. It activates three different G-protein-coupled receptors known as BBR1, -2, and -3. It also activates these receptors in the brain. Together with cholecystokinin, it is the second major source of negative feedback signals that stop eating behaviour.

Gastrin-releasing peptide, also known as GRP, is a neuropeptide, a regulatory molecule that has been implicated in a number of physiological and pathophysiological processes. Most notably, GRP stimulates the release of gastrin from the G cells of the stomach.
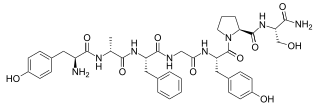
Dermorphin is a hepta-peptide first isolated from the skin of South American frogs belonging to the genus Phyllomedusa. The peptide is a natural opioid that binds as an agonist with high potency and selectivity to mu Opioid receptors. Dermorphin is about 30–40 times more potent than morphine but theoretically may be less likely to produce drug tolerance and addiction (due to its high potency). The amino acid sequence of dermorphin is H-Tyr-D-Ala-Phe-Gly-Tyr-Pro-Ser-NH2.

Physalaemin is a tachykinin peptide obtained from the Physalaemus frog, closely related to substance P. Its structure was first elucidated in 1964.
The bombesin receptors are a group of G-protein coupled receptors which bind bombesin.
Three bombesin receptors are currently known:
BB1, previously known as Neuromedin B receptor NMBR
BB2, previously known as Gastrin-releasing peptide receptor GRPR
BB3, previously known as Bombesin-like receptor 3 BRS3

Neurokinin A (NKA), formerly known as Substance K, is a neurologically active peptide translated from the pre-protachykinin gene. Neurokinin A has many excitatory effects on mammalian nervous systems and is also influential on the mammalian inflammatory and pain responses.
Neuromedin B (NMB) is a bombesin-related peptide in mammals. It was originally purified from pig spinal cord, and later shown to be present in human central nervous system and gastrointestinal tract.

The bombesin receptor subtype 3 also known as BRS-3 or BB3 is a protein which in humans is encoded by the BRS3 gene.

The neuromedin B receptor (NMBR), now known as BB1 is a G protein-coupled receptor whose endogenous ligand is neuromedin B. In humans, this protein is encoded by the NMBR gene.

The gastrin-releasing peptide receptor (GRPR), now properly known as BB2 is a G protein-coupled receptor whose endogenous ligand is gastrin releasing peptide. In humans it is highly expressed in the pancreas and is also expressed in the stomach, adrenal cortex and brain.
Neuromedin U is a neuropeptide found in the brain of humans and other mammals, which has a number of diverse functions including contraction of smooth muscle, regulation of blood pressure, pain perception, appetite, bone growth, and hormone release. It was first isolated from the spinal cord in 1985, and named after its ability to cause smooth muscle contraction in the uterus.

N-formyl peptide receptor 3 (FPR3) is a receptor protein that in humans is encoded by the FPR3 gene.

Neuromedin-U receptor 2 is a protein that in humans is encoded by the NMUR2 gene.

Amelanism is a pigmentation abnormality characterized by the lack of pigments called melanins, commonly associated with a genetic loss of tyrosinase function. Amelanism can affect fish, amphibians, reptiles, birds, and mammals including humans. The appearance of an amelanistic animal depends on the remaining non-melanin pigments. The opposite of amelanism is melanism, a higher percentage of melanin.
The gastrin family of proteins is defined by the peptide hormones gastrin and cholecystokinin. Gastrin and cholecystokinin (CCK) are structurally and functionally related peptide hormones that serve as regulators of various digestive processes and feeding behaviors. Additional structurally related members of this family include the amphibian caerulein skin peptide, the cockroach leukosulphakinin I and II (LSK) peptides, Drosophila melanogaster putative CCK-homologs Drosulphakinins I and II, cionin, a chicken gastrin/cholecystokinin-like peptide and cionin, a neuropeptide from the protochordate Ciona intestinalis.
Deltorphin, also known as deltorphin A and dermenkephalin, is a naturally occurring, exogenous opioid heptapeptide and thus, exorphin, with the amino acid sequence Tyr-D-Met-Phe-His-Leu-Met-Asp-NH2. Along with the other deltorphins (such as deltorphin I and deltorphin II) and the dermorphins, deltorphin is endogenous to frogs of the genus Phyllomedusa such as P. bicolor and P. sauvagei where it is produced in their skin, and is not known to occur naturally in any other species. Deltorphin is one of the highest affinity and most selective naturally occurring opioid peptides known, acting as a very potent and highly specific agonist of the δ-opioid receptor.
Sauvagine is a neuropeptide from the corticotropin-releasing factor (CRF) family of peptides and is orthologous to the mammalian hormone, urocortin 1, and the teleost fish hormone, urotensin 1. It is 40 amino acids in length, and has the sequence XGPPISIDLSLELLRKMIEIEKQEKEKQQAANNRLLLDTI-NH2, with a pyrrolidone carboxylic acid modification at the N-terminal and amidation of the C-terminal isoleucine residue. It was originally isolated from the skin of the frog Phyllomedusa sauvagei. Given its relation to other CRF-related peptides, it exerts similar physiological effects as corticotropin-releasing hormone.
Pro-Gastrin-Releasing-Peptide, also known as Pro-GRP, is a Gastrin-Releasing-Peptide (GRP) precursor, a neurotransmitter that belongs to the bombesine/neuromedin B family. GRP stimulates the secretion of gastrin in order to increase the acidity of the gastric acid. Pro-GRP is a peptide composed of 125 amino acids, expressed in the nervous system and digestive tract. It is also important to not confused with progastrin, consisting of 80 amino acids, precursor of gastrin in its intracellular version and oncogene in its extracellular version (hPG80).
Progastrin is an 80-amino acid intracellular protein and the precursor of gastrin, a gastrointestinal hormone produced by G cells in the gastric antrum. The main function of gastrin is to regulate acid secretion. During digestion, only gastrin is released into the bloodstream and stimulates the secretion of hydrochloric acid in the stomach as well as pancreatic digestive enzymes. In humans, progastrin is encoded by the GAST gene. Progastrin is expressed primarily in stomach tissue.
References
- ↑ Erspamer V, Erspamer GF, Mazzanti G, Endean R (1984). "Active peptides in the skins of one hundred amphibian species from Australia and Papua New Guinea". Comp. Biochem. Physiol. 77 (1): 99–108. doi:10.1016/0742-8413(84)90137-3. PMID 6141890.
- ↑ Erspamer V, Melchiorri P, Falconieri Erspamer G, Montecucchi PC, de Castiglione R (1985). "Phyllomedusa skin: a huge factory and store-house of a variety of active peptides". Peptides. 6: 7–12. doi:10.1016/0196-9781(85)90343-2. PMID 3868775. S2CID 3851448.
- ↑ Battey J, Wada E (1991). "Two distinct receptor subtypes for mammalian bombesin-like peptides". Trends Neurosci. 14 (12): 524–528. doi:10.1016/0166-2236(91)90005-F. PMID 1726343. S2CID 4029842.
- ↑ Chin WW, Krane IM, Naylor SL, Helin-Davis D, Spindel ER (1988). "Molecular cloning of cDNAs encoding the human bombesin-like peptide neuromedin B. Chromosomal localization and comparison to cDNAs encoding its amphibian homolog ranatensin". J. Biol. Chem. 263 (26): 13317–13323. PMID 2458345.