
Boron is a chemical element; it has symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the boron group it has three valence electrons for forming covalent bonds, resulting in many compounds such as boric acid, the mineral sodium borate, and the ultra-hard crystals of boron carbide and boron nitride.

In nuclear physics, a nuclear chain reaction occurs when one single nuclear reaction causes an average of one or more subsequent nuclear reactions, thus leading to the possibility of a self-propagating series or "positive feedback loop" of these reactions. The specific nuclear reaction may be the fission of heavy isotopes. A nuclear chain reaction releases several million times more energy per reaction than any chemical reaction.
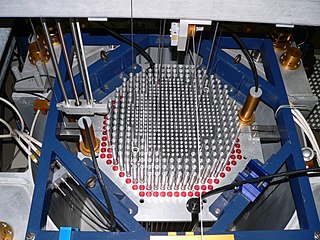
A nuclear reactor is a device used to initiate and control a fission nuclear chain reaction or nuclear fusion reactions. Nuclear reactors are used at nuclear power plants for electricity generation and in nuclear marine propulsion. Heat from nuclear fission is passed to a working fluid, which in turn runs through steam turbines. These either drive a ship's propellers or turn electrical generators' shafts. Nuclear generated steam in principle can be used for industrial process heat or for district heating. Some reactors are used to produce isotopes for medical and industrial use, or for production of weapons-grade plutonium. As of 2022, the International Atomic Energy Agency reports there are 422 nuclear power reactors and 223 nuclear research reactors in operation around the world.

A pressurized water reactor (PWR) is a type of light-water nuclear reactor. PWRs constitute the large majority of the world's nuclear power plants. In a PWR, the primary coolant (water) is pumped under high pressure to the reactor core where it is heated by the energy released by the fission of atoms. The heated, high pressure water then flows to a steam generator, where it transfers its thermal energy to lower pressure water of a secondary system where steam is generated. The steam then drives turbines, which spin an electric generator. In contrast to a boiling water reactor (BWR), pressure in the primary coolant loop prevents the water from boiling within the reactor. All light-water reactors use ordinary water as both coolant and neutron moderator. Most use anywhere from two to four vertically mounted steam generators; VVER reactors use horizontal steam generators.

In nuclear engineering, a neutron moderator is a medium that reduces the speed of fast neutrons, ideally without capturing any, leaving them as thermal neutrons with only minimal (thermal) kinetic energy. These thermal neutrons are immensely more susceptible than fast neutrons to propagate a nuclear chain reaction of uranium-235 or other fissile isotope by colliding with their atomic nucleus.
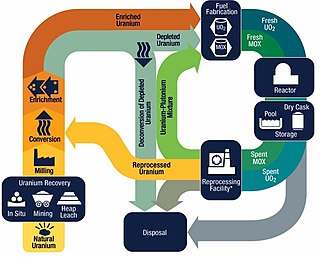
The nuclear fuel cycle, also called nuclear fuel chain, is the progression of nuclear fuel through a series of differing stages. It consists of steps in the front end, which are the preparation of the fuel, steps in the service period in which the fuel is used during reactor operation, and steps in the back end, which are necessary to safely manage, contain, and either reprocess or dispose of spent nuclear fuel. If spent fuel is not reprocessed, the fuel cycle is referred to as an open fuel cycle ; if the spent fuel is reprocessed, it is referred to as a closed fuel cycle.

A breeder reactor is a nuclear reactor that generates more fissile material than it consumes. These reactors can be fueled with more-commonly available isotopes of uranium and thorium, such as uranium-238 and thorium-232, as opposed to the rare uranium-235 which is used in conventional reactors. These materials are called fertile materials since they can be bred into fuel by these breeder reactors.

Control rods are used in nuclear reactors to control the rate of fission of the nuclear fuel – uranium or plutonium. Their compositions include chemical elements such as boron, cadmium, silver, hafnium, or indium, that are capable of absorbing many neutrons without themselves decaying. These elements have different neutron capture cross sections for neutrons of various energies. Boiling water reactors (BWR), pressurized water reactors (PWR), and heavy-water reactors (HWR) operate with thermal neutrons, while breeder reactors operate with fast neutrons. Each reactor design can use different control rod materials based on the energy spectrum of its neutrons. Control rods have been used in nuclear aircraft engines like Project Pluto as a method of control.

A fast-neutron reactor (FNR) or fast-spectrum reactor or simply a fast reactor is a category of nuclear reactor in which the fission chain reaction is sustained by fast neutrons, as opposed to slow thermal neutrons used in thermal-neutron reactors. Such a fast reactor needs no neutron moderator, but requires fuel that is relatively rich in fissile material when compared to that required for a thermal-neutron reactor. Around 20 land based fast reactors have been built, accumulating over 400 reactor years of operation globally. The largest was the Superphénix sodium cooled fast reactor in France that was designed to deliver 1,242 MWe. Fast reactors have been studied since the 1950s, as they provide certain advantages over the existing fleet of water-cooled and water-moderated reactors. These are:

The light-water reactor (LWR) is a type of thermal-neutron reactor that uses normal water, as opposed to heavy water, as both its coolant and neutron moderator; furthermore a solid form of fissile elements is used as fuel. Thermal-neutron reactors are the most common type of nuclear reactor, and light-water reactors are the most common type of thermal-neutron reactor.
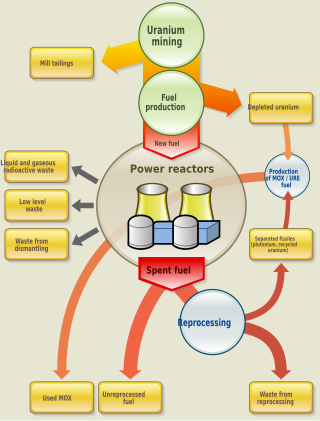
Nuclear fuel is material used in nuclear power stations to produce heat to power turbines. Heat is created when nuclear fuel undergoes nuclear fission. Nuclear fuel has the highest energy density of all practical fuel sources. The processes involved in mining, refining, purifying, using, and disposing of nuclear fuel are collectively known as the nuclear fuel cycle.

Trevorite is a rare nickel iron oxide mineral belonging to the spinel group. It has the chemical formula NiFe3+2O4. It is a black mineral with the typical spinel properties of crystallising in the cubic system, black streaked, infusible and insoluble in most acids.

A reactor pressure vessel (RPV) in a nuclear power plant is the pressure vessel containing the nuclear reactor coolant, core shroud, and the reactor core.

The supercritical water reactor (SCWR) is a concept Generation IV reactor, designed as a light water reactor (LWR) that operates at supercritical pressure. The term critical in this context refers to the critical point of water, and should not be confused with the concept of criticality of the nuclear reactor.
Generation IVreactors are nuclear reactor design technologies that are envisioned as successors of generation III reactors. The Generation IV International Forum (GIF) – an international organization that coordinates the development of generation IV reactors – specifically selected six reactor technologies as candidates for generation IV reactors. The designs target improved safety, sustainability, efficiency, and cost. The World Nuclear Association in 2015 suggested that some might enter commercial operation before 2030.
In applications such as nuclear reactors, a neutron poison is a substance with a large neutron absorption cross-section. In such applications, absorbing neutrons is normally an undesirable effect. However, neutron-absorbing materials, also called poisons, are intentionally inserted into some types of reactors in order to lower the high reactivity of their initial fresh fuel load. Some of these poisons deplete as they absorb neutrons during reactor operation, while others remain relatively constant.
The three primary objectives of nuclear reactor safety systems as defined by the U.S. Nuclear Regulatory Commission are to shut down the reactor, maintain it in a shutdown condition and prevent the release of radioactive material.

FLiBe is the name of a molten salt made from a mixture of lithium fluoride (LiF) and beryllium fluoride. It is both a nuclear reactor coolant and solvent for fertile or fissile material. It served both purposes in the Molten-Salt Reactor Experiment (MSRE) at the Oak Ridge National Laboratory.
A nuclear reactor coolant is a coolant in a nuclear reactor used to remove heat from the nuclear reactor core and transfer it to electrical generators and the environment. Frequently, a chain of two coolant loops are used because the primary coolant loop takes on short-term radioactivity from the reactor.

Bunsenite is the naturally occurring form of nickel(II) oxide, NiO. It occurs as rare dark green crystal coatings. It crystallizes in the cubic crystal system and occurs as well formed cubic, octahedral and dodecahedral crystals. It is a member of the periclase group.
















