
In chemistry, a carbide usually describes a compound composed of carbon and a metal. In metallurgy, carbiding or carburizing is the process for producing carbide coatings on a metal piece.
A metalloid is a chemical element which has a preponderance of properties in between, or that are a mixture of, those of metals and nonmetals. The word metalloid comes from the Latin metallum ("metal") and the Greek oeides. There is no standard definition of a metalloid and no complete agreement on which elements are metalloids. Despite the lack of specificity, the term remains in use in the literature.

In the context of the periodic table a nonmetal is a chemical element that mostly lacks distinctive metallic properties. They range from colorless gases like hydrogen to shiny crystals like iodine. Physically, they are usually lighter than elements that form metals and are often poor conductors of heat and electricity. Chemically, nonmetals have relatively high electronegativity or usually attract electrons in a chemical bond with another element, and their oxides tend to be acidic.

Boron hydride clusters are compounds with the formula BxHy or related anions, where x ≥ 3. Many such cluster compounds are known. Common examples are those with 5, 10, and 12 boron atoms. Although they have few practical applications, the borane hydride clusters exhibit structures and bonding that differs strongly from the patterns seen in hydrocarbons. Hybrids of boranes and hydrocarbons, the carboranes are also well developed.

Diborane(6), commonly known as diborane, is the chemical compound with the formula B2H6. It is a highly toxic, colorless, and pyrophoric gas with a repulsively sweet odor. Given its simple formula, borane is a fundamental boron compound. It has attracted wide attention for its electronic structure. Several of its derivatives are useful reagents.

Decaborane, also called decaborane(14), is the inorganic compound with the chemical formula B10H14. It is classified as a borane and more specifically a boron hydride cluster. This white crystalline compound is one of the principal boron hydride clusters, both as a reference structure and as a precursor to other boron hydrides. It is toxic and volatile, giving off a foul odor, like that of burnt rubber or chocolate.
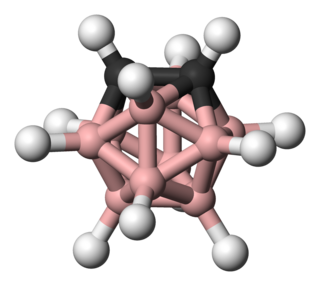
Carboranes are electron-delocalized clusters composed of boron, carbon and hydrogen atoms. Like many of the related boron hydrides, these clusters are polyhedra or fragments of polyhedra. Carboranes are one class of heteroboranes.
Boron trifluoride is the inorganic compound with the formula BF3. This pungent, colourless, and toxic gas forms white fumes in moist air. It is a useful Lewis acid and a versatile building block for other boron compounds.
A boride is a compound between boron and a less electronegative element, for example silicon boride (SiB3 and SiB6). The borides are a very large group of compounds that are generally high melting and are covalent more than ionic in nature. Some borides exhibit very useful physical properties. The term boride is also loosely applied to compounds such as B12As2 (N.B. Arsenic has an electronegativity higher than boron) that is often referred to as icosahedral boride.

Borazine, also known as borazole, is an inorganic compound with the chemical formula B3H6N3. In this cyclic compound, the three BH units and three NH units alternate. The compound is isoelectronic and isostructural with benzene. For this reason borazine is sometimes referred to as “inorganic benzene”. Like benzene, borazine is a colourless liquid with an aromatic odor.
In chemistry the polyhedral skeletal electron pair theory (PSEPT) provides electron counting rules useful for predicting the structures of clusters such as borane and carborane clusters. The electron counting rules were originally formulated by Kenneth Wade, and were further developed by others including Michael Mingos; they are sometimes known as Wade's rules or the Wade–Mingos rules. The rules are based on a molecular orbital treatment of the bonding. These rules have been extended and unified in the form of the Jemmis mno rules.

1,1′-Bis(diphenylphosphino)ferrocene, commonly abbreviated dppf, is an organophosphorus compound commonly used as a ligand in homogeneous catalysis. It contains a ferrocene moiety in its backbone, and is related to other bridged diphosphines such as 1,2-bis(diphenylphosphino)ethane (dppe).

Boron can be prepared in several crystalline and amorphous forms. Well known crystalline forms are α-rhombohedral (α-R), β-rhombohedral (β-R), and β-tetragonal (β-T). In special circumstances, boron can also be synthesized in the form of its α-tetragonal (α-T) and γ-orthorhombic (γ) allotropes. Two amorphous forms, one a finely divided powder and the other a glassy solid, are also known. Although at least 14 more allotropes have been reported, these other forms are based on tenuous evidence or have not been experimentally confirmed, or are thought to represent mixed allotropes, or boron frameworks stabilized by impurities. Whereas the β-rhombohedral phase is the most stable and the others are metastable, the transformation rate is negligible at room temperature, and thus all five phases can exist at ambient conditions. Amorphous powder boron and polycrystalline β-rhombohedral boron are the most common forms. The latter allotrope is a very hard grey material, about ten percent lighter than aluminium and with a melting point (2080 °C) several hundred degrees higher than that of steel.
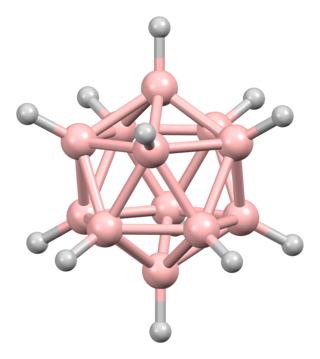
The dodecaborate(12) anion, [B12H12]2−, is a borane with an icosahedral arrangement of 12 boron atoms, with each boron atom being attached to a hydrogen atom. Its symmetry is classified by the molecular point group Ih.

1,2-Dimethyldiborane is an organoboron compound with the formula [(CH3)BH2]2. Structurally, it is related to diborane, but with methyl groups replacing terminal hydrides on each boron. It is the dimer of methylborane, CH3BH2, the simplest alkylborane. 1,2-Dimethyldiborane can exist in a cis- and a trans arrangement. 1,2-Dimethyldiborane is an easily condensed, colorless gas that ignites spontaneously in air.
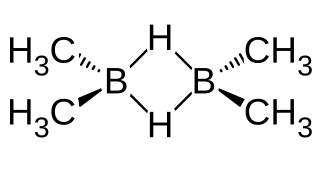
Dimethylborane, (CH3)2BH is the simplest dialkylborane, consisting of a methyl group substituted for a hydrogen in borane. As for other boranes it normally exists in the form of a dimer called tetramethyldiborane or tetramethylbisborane or TMDB ((CH3)2BH)2. Other combinations of methylation occur on diborane, including monomethyldiborane, trimethyldiborane, 1,2-dimethylborane, 1,1-dimethylborane and trimethylborane. At room temperature the substance is at equilibrium between these forms. The methylboranes were first prepared by H. I. Schlesinger and A. O. Walker in the 1930s.

Trimethyldiborane, (CH3)3B2H3 is a molecule containing boron carbon and hydrogen. It is an alkylborane, consisting of three methyl group substituted for a hydrogen in diborane. It can be considered a mixed dimer: (CH3)2BH2BH(CH3) or dimethylborane and methylborane. called 1,2-dimethyldiborane. Other combinations of methylation occur on diborane, including monomethyldiborane, 1,2-dimethyldiborane, tetramethyldiborane, 1,1-dimethylborane and trimethylborane. At room temperature the substance is at equilibrium between these forms, so it is difficult to keep it pure. The methylboranes were first prepared by H. I. Schlesinger and A. O. Walker in the 1930s.
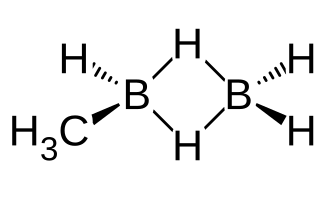
Methyldiborane, CH3B2H5, or monomethyldiborane is the simplest of alkyldiboranes, consisting of a methyl group substituted for a hydrogen in diborane. As with other boranes it exists in the form of a dimer with a twin hydrogen bridge that uses three-center two-electron bonding between the two boron atoms, and can be imagined as methyl borane (CH3BH2) bound to borane (BH3). Other combinations of methylation occur on diborane, including 1,1-dimethylborane, 1,2-dimethyldiborane, trimethyldiborane, tetramethyldiborane, and trimethylborane (which is not a dimer). At room temperature the substance is at equilibrium between these molecules.

1,1-Dimethyldiborane is the organoboron compound with the formula (CH3)2B(μ-H)2BH2. A pair of related 1,2-dimethyldiboranes are also known. It is a colorless gas that ignites in air.

ortho-Carborane is the organoboron compound with the formula C2B10H12. The prefix ortho is derived from ortho. It is the most prominent carborane. This derivative has been considered for a wide range of applications from heat-resistant polymers to medical applications. It is a colorless solid that melts, without decomposition, at 320 °C














