
Acetoacetic acid is the organic compound with the formula CH3COCH2COOH. It is the simplest beta-keto acid, and like other members of this class, it is unstable. The methyl and ethyl esters, which are quite stable, are produced on a large scale industrially as precursors to dyes. Acetoacetic acid is a weak acid.

Frankincense, also known as olibanum, is an aromatic resin used in incense and perfumes, obtained from trees of the genus Boswellia in the family Burseraceae. The word is from Old French franc encens. There are several species of Boswellia that produce true frankincense: Boswellia sacra, B. frereana, B. serrata, and B. papyrifera. Resin from each is available in various grades, which depends on the time of harvesting. The resin is hand-sorted for quality.

Eicosanoids are signaling molecules made by the enzymatic or non-enzymatic oxidation of arachidonic acid or other polyunsaturated fatty acids (PUFAs) that are, similar to arachidonic acid, around 20 carbon units in length. Eicosanoids are a sub-category of oxylipins, i.e. oxidized fatty acids of diverse carbon units in length, and are distinguished from other oxylipins by their overwhelming importance as cell signaling molecules. Eicosanoids function in diverse physiological systems and pathological processes such as: mounting or inhibiting inflammation, allergy, fever and other immune responses; regulating the abortion of pregnancy and normal childbirth; contributing to the perception of pain; regulating cell growth; controlling blood pressure; and modulating the regional flow of blood to tissues. In performing these roles, eicosanoids most often act as autocrine signaling agents to impact their cells of origin or as paracrine signaling agents to impact cells in the proximity of their cells of origin. Some eicosanoids, such as prostaglandins, may also have endocrine roles as hormones to influence the function of distant cells.

Boswellia is a genus of trees in the order Sapindales, known for its fragrant resin. The biblical incense frankincense is an extract from the resin of the tree Boswellia sacra, and is now produced also from B. frereana. Boswellia species are moderate-sized flowering plants, including both trees and shrubs.
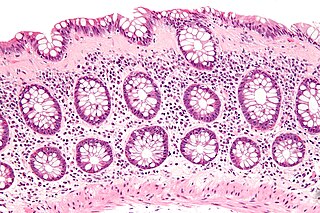
Collagenous colitis is an inflammatory condition of the colon. Together with the related condition lymphocytic colitis, it is a subtype of microscopic colitis, which is characterized by inflammation that specifically affects the colon, and a clinical presentation that involves watery diarrhea but a lack of rectal bleeding. Microscopic colitis does not usually cause macroscopic changes to the colon that allow a visual diagnosis during colonoscopy, instead causing microscopic changes that can be detected through histopathological examination of colonic biopsies. The nature of these microscopic changes is what differentiates collagenous from lymphocytic colitis, with the characteristic finding in collagenous colitis being depositions of collagen in the connective tissue between the colonic glands. Collagenous colitis, and microscopic colitis as a whole, is sometimes considered to be an inflammatory bowel disease (IBD) along with Crohn's disease and ulcerative colitis. However, little is known about the etiology of microscopic colitis, and so the degree of similarity to the inflammatory bowel diseases is uncertain.
In biochemistry, fatty acid synthesis is the creation of fatty acids from acetyl-CoA and NADPH through the action of enzymes called fatty acid synthases. This process takes place in the cytoplasm of the cell. Most of the acetyl-CoA which is converted into fatty acids is derived from carbohydrates via the glycolytic pathway. The glycolytic pathway also provides the glycerol with which three fatty acids can combine to form triglycerides, the final product of the lipogenic process. When only two fatty acids combine with glycerol and the third alcohol group is phosphorylated with a group such as phosphatidylcholine, a phospholipid is formed. Phospholipids form the bulk of the lipid bilayers that make up cell membranes and surrounds the organelles within the cells. In addition to cytosolic fatty acid synthesis, there is also mitochondrial fatty acid synthesis (mtFASII), in which malonyl-CoA is formed from malonic acid with the help of malonyl-CoA synthetase (ACSF3), which then becomes the final product octanoyl-ACP (C8) via further intermediate steps.
Arachidonate 5-lipoxygenase, also known as ALOX5, 5-lipoxygenase, 5-LOX, or 5-LO, is a non-heme iron-containing enzyme that in humans is encoded by the ALOX5 gene. Arachidonate 5-lipoxygenase is a member of the lipoxygenase family of enzymes. It transforms essential fatty acids (EFA) substrates into leukotrienes as well as a wide range of other biologically active products. ALOX5 is a current target for pharmaceutical intervention in a number of diseases.

Maslinic acid is a compound derived from dry olive-pomace oil which is a byproduct of olive oil extraction. It is a member of the group of triterpenes known as oleananes.

Boswellia sacra, also known as Boswellia carteri and others, and commonly called the frankincense tree or the olibanum tree, is a tree in the genus Boswellia, in the Burseraceae family, from which frankincense, a resinous dried sap, is harvested. The olibanum tree is plant native to the countries of Oman and Yemen, in the south of the Arabian Peninsula, and to Somalia, in the Horn of Africa.

Boswellia serrata is a plant that produces Indian frankincense. The plant is native to much of India and the Punjab region that extends into Pakistan.

Boswellia papyrifera, also known as the Sudanese frankincense, is a species of flowering plant and frankincense that is native to Ethiopia, Eritrea and Sudan. The tree is cultivated in Ethiopia because of its valuable resin. The incense is characterized by a fresh lemon-pine scent and is therefore highly esteemed. In Ethiopia where it is called itan zaf, it comes in semi-translucent yellow tears. The gum resin of Boswellia papyrifera coming from Ethiopia, Sudan and eastern Africa is believed to be the main source of frankincense of antiquity.
Arachidonate 5-lipoxygenase inhibitors are compounds that slow or stop the action of the arachidonate 5-lipoxygenase enzyme, which is responsible for the production of inflammatory leukotrienes. The overproduction of leukotrienes is a major cause of inflammation in asthma, allergic rhinitis, and osteoarthritis.

ALOX15 is, like other lipoxygenases, a seminal enzyme in the metabolism of polyunsaturated fatty acids to a wide range of physiologically and pathologically important products. ▼ Gene Function
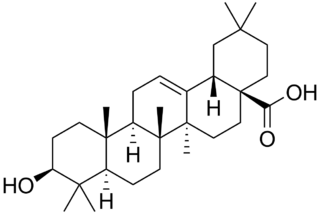
Oleanolic acid or oleanic acid is a naturally occurring pentacyclic triterpenoid related to betulinic acid. It is widely distributed in food and plants where it exists as a free acid or as an aglycone of triterpenoid saponins.
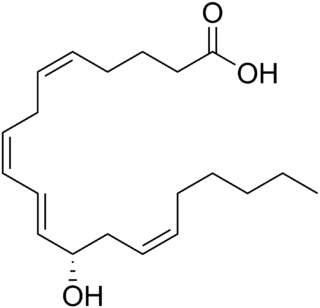
12-Hydroxyeicosatetraenoic acid (12-HETE) is a derivative of the 20 carbon polyunsaturated fatty acid, arachidonic acid, containing a hydroxyl residue at carbon 12 and a 5Z,8Z,10E,14Z Cis–trans isomerism configuration (Z=cis, E=trans) in its four double bonds. It was first found as a product of arachidonic acid metabolism made by human and bovine platelets through their 12S-lipoxygenase (i.e. ALOX12) enzyme(s). However, the term 12-HETE is ambiguous in that it has been used to indicate not only the initially detected "S" stereoisomer, 12S-hydroxy-5Z,8Z,10E,14Z-eicosatetraenoic acid (12(S)-HETE or 12S-HETE), made by platelets, but also the later detected "R" stereoisomer, 12(R)-hydroxy-5Z,8Z,10E,14Z-eicosatetraenoic acid (also termed 12(R)-HETE or 12R-HETE) made by other tissues through their 12R-lipoxygenase enzyme, ALOX12B. The two isomers, either directly or after being further metabolized, have been suggested to be involved in a variety of human physiological and pathological reactions. Unlike hormones which are secreted by cells, travel in the circulation to alter the behavior of distant cells, and thereby act as Endocrine signalling agents, these arachidonic acid metabolites act locally as Autocrine signalling and/or Paracrine signaling agents to regulate the behavior of their cells of origin or of nearby cells, respectively. In these roles, they may amplify or dampen, expand or contract cellular and tissue responses to disturbances.
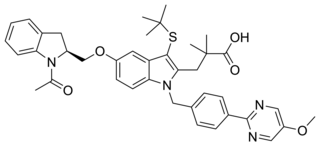
AM-679 is a drug which acts as a selective inhibitor of 5-Lipoxygenase-activating protein (FLAP). This protein is involved in the production of cysteinyl leukotrienes which are involved in inflammation, and AM-679 has anti-inflammatory effects in animal studies.
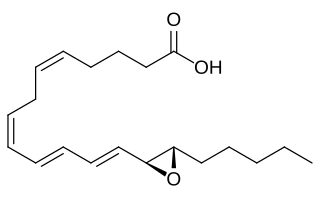
Eoxins are proposed to be a family of proinflammatory eicosanoids. They are produced by human eosinophils, mast cells, the L1236 Reed–Sternberg cell line derived from Hodgkin's lymphoma, and certain other tissues. These cells produce the eoxins by initially metabolizing arachidonic acid, an omega-6 (ω-6) fatty acid, via any enzyme possessing 15-lipoxygenase activity. The product of this initial metabolic step, 15(S)-hydroperoxyeicosatetraenoic acid, is then converted to a series of eoxins by the same enzymes that metabolize the 5-lipoxygenase product of arachidonic acid metabolism, i.e. 5-Hydroperoxy-eicosatetraenoic acid to a series of leukotrienes. That is, the eoxins are 14,15-disubstituted analogs of the 5,6-disubstituted leukotrienes.
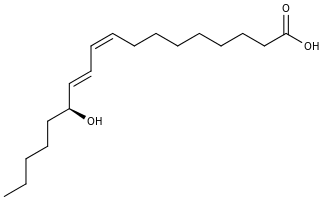
13-Hydroxyoctadecadienoic acid (13-HODE) is the commonly used term for 13(S)-hydroxy-9Z,11E-octadecadienoic acid (13(S)-HODE). The production of 13(S)-HODE is often accompanied by the production of its stereoisomer, 13(R)-hydroxy-9Z,11E-octadecadienoic acid (13(R)-HODE). The adjacent figure gives the structure for the (S) stereoisomer of 13-HODE. Two other naturally occurring 13-HODEs that may accompany the production of 13(S)-HODE are its cis-trans (i.e., 9E,11E) isomers viz., 13(S)-hydroxy-9E,11E-octadecadienoic acid (13(S)-EE-HODE) and 13(R)-hydroxy-9E,11E-octadecadienoic acid (13(R)-EE-HODE). Studies credit 13(S)-HODE with a range of clinically relevant bioactivities; recent studies have assigned activities to 13(R)-HODE that differ from those of 13(S)-HODE; and other studies have proposed that one or more of these HODEs mediate physiological and pathological responses, are markers of various human diseases, and/or contribute to the progression of certain diseases in humans. Since, however, many studies on the identification, quantification, and actions of 13(S)-HODE in cells and tissues have employed methods that did not distinguish between these isomers, 13-HODE is used here when the actual isomer studied is unclear.

Incensole is a C20 diterpene alcohol and biomarker for some plants of the Boswellia genus. It, along with its acetate ester incensole acetate, is an abundant component of frankincense, the resin collected from Boswellia trees. Incensole is used archaeologically to assist in identifying trade routes and distinguishing the identity of frankincense from other resins which may have been used together in incense and other salves. Incensole has also been deemed to be an active component in medicinal frankincense.
Ahmed Sulaiman Al-Harrasi is an Omani scientist and a professor of organic chemistry at the University of Nizwa, Nizwa, Sultanate of Oman.





















