
Cobalt(II) chloride is an inorganic compound of cobalt and chlorine, with the formula CoCl
2. It is a sky blue crystalline solid.

Nickel(II) chloride (or just nickel chloride), is the chemical compound NiCl2. The anhydrous salt is yellow, but the more familiar hydrate NiCl2·6H2O is green. Nickel(II) chloride, in various forms, is the most important source of nickel for chemical synthesis. The nickel chlorides are deliquescent, absorbing moisture from the air to form a solution. Nickel salts have been shown to be carcinogenic to the lungs and nasal passages in cases of long-term inhalation exposure.

Iron(II) chloride, also known as ferrous chloride, is the chemical compound of formula FeCl2. It is a paramagnetic solid with a high melting point. The compound is white, but typical samples are often off-white. FeCl2 crystallizes from water as the greenish tetrahydrate, which is the form that is most commonly encountered in commerce and the laboratory. There is also a dihydrate. The compound is highly soluble in water, giving pale green solutions.
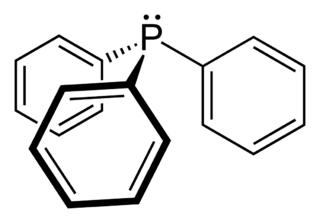
Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 - often abbreviated to PPh3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists as relatively air stable, colorless crystals at room temperature. It dissolves in non-polar organic solvents such as benzene and diethyl ether.

In coordination chemistry, metal ammine complexes are metal complexes containing at least one ammonia (NH3) ligand. "Ammine" is spelled this way due to historical reasons; in contrast, alkyl or aryl bearing ligands are spelt with a single "m". Almost all metal ions bind ammonia as a ligand, but the most prevalent examples of ammine complexes are for Cr(III), Co(III), Ni(II), Cu(II) as well as several platinum group metals.

Hexol is the name for various salts of a coordination complex that has historical significance. The salts were the first synthetic non-carbon-containing chiral compounds. The sulfate salt has the formula {[Co(NH3)4(OH)2]3Co}(SO4)3.

Hexaamminecobalt(III) chloride is the chemical compound with the formula [Co(NH3)6]Cl3. It is the chloride salt of the coordination complex [Co(NH3)6]3+, which is considered an archetypal "Werner complex", named after the pioneer of coordination chemistry, Alfred Werner. The cation itself is a metal ammine complex with six ammonia ligands attached to the cobalt(III) ion.

Bromopentacarbonylrhenium(I) is an inorganic compound of rhenium, commonly used for the syntheses of other rhenium complexes.

Cobalt(II) bromide (CoBr2) is an inorganic compound. In its anhydrous form, it is a green solid that is soluble in water, used primarily as a catalyst in some processes.

In chemistry, a template reaction is any of a class of ligand-based reactions that occur between two or more adjacent coordination sites on a metal center. In the absence of the metal ion, the same organic reactants produce different products. The term is mainly used in coordination chemistry. The template effects emphasizes the pre-organization provided by the coordination sphere, although the coordination modifies the electronic properties of ligands.
Organogold chemistry is the study of compounds containing gold–carbon bonds. They are studied in academic research, but have not received widespread use otherwise. The dominant oxidation states for organogold compounds are I with coordination number 2 and a linear molecular geometry and III with CN = 4 and a square planar molecular geometry. The first organogold compound discovered was gold(I) carbide Au2C2, which was first prepared in 1900.
Organorhenium chemistry describes the compounds with Re−C bonds. Because rhenium is a rare element, relatively few applications exist, but the area has been a rich source of concepts and a few useful catalysts.

Metal amides (systematic name metal azanides) are a class of coordination compounds composed of a metal center with amide ligands of the form NR2−. Amide ligands have two electron pairs available for bonding. In principle, they can be terminal or bridging. In these two examples, the dimethylamido ligands are both bridging and terminal:

A metal salen complex is a coordination compound between a metal cation and a ligand derived from N,N′-bis(salicylidene)ethylenediamine, commonly called salen. The classical example is salcomine, the complex with divalent cobalt Co2+, usually denoted as Co(salen). These complexes are widely investigated as catalysts and enzyme mimics.

Metal halides are compounds between metals and halogens. Some, such as sodium chloride are ionic, while others are covalently bonded. Covalently bonded metal halides may be discrete molecules, such as uranium hexafluoride, or they may form polymeric structures, such as palladium chloride.

Chloropentamminecobalt chloride is the dichloride salt of the coordination complex [Co(NH3)5Cl]2+. It is a red-violet, diamagnetic, water-soluble salt. The compound has been of academic and historical interest.
Compounds of nickel are chemical compounds containing the element nickel which is a member of the group 10 of the periodic table. Most compounds in the group have an oxidation state of +2. Nickel is classified as a transition metal with nickel(II) having much chemical behaviour in common with iron(II) and cobalt(II). Many salts of nickel(II) are isomorphous with salts of magnesium due to the ionic radii of the cations being almost the same. Nickel forms many coordination complexes. Nickel tetracarbonyl was the first pure metal carbonyl produced, and is unusual in its volatility. Metalloproteins containing nickel are found in biological systems.
Cobalt(II)–porphyrin catalysis is a process in which a Co(II) porphyrin complex acts as a catalyst, inducing and accelerating a chemical reaction.

Transition metal nitrile complexes are coordination compounds containing nitrile ligands. Because nitriles are weakly basic, the nitrile ligands in these complexes are often labile.

In chemistry, a transition metal chloride complex is a coordination complex that consists of a transition metal coordinated to one or more chloride ligand. The class of complexes is extensive.
















