
Bruno Chaudret, born on 25 December 1953, is a French chemist and director of research at the CNRS. His research is in organometallic chemistry, particularly the interactions between hydrogen and transition metals.

Bruno Chaudret, born on 25 December 1953, is a French chemist and director of research at the CNRS. His research is in organometallic chemistry, particularly the interactions between hydrogen and transition metals.
Chaudret is a graduate of the École Nationale Supérieure de Chimie de Paris (1975). He then completed a Ph.D. at Imperial College in London (1977) under Sir Geoffrey Wilkinson, and a Ph.D. at the Université Paul Sabatier in Toulouse (1979) under Professor René Poilblanc. He joined the CNRS as a research associate in 1977, and is now Director of Exceptional Class Research at the CNRS and Director of the Laboratory of Physics and Chemistry of Nano-Objects at INSA in Toulouse (UMR 5215 INSA-CNRS-UPS). [1]
Chaudret synthesized the first bis(dihydrogen) complex and demonstrated its high reactivity, particularly for the activation of C-H, Si-H and more generally of poorly reactive bonds. [2] He has also been interested in the spectroscopic properties of these species, in particular the quantum exchange of protons in the coordination sphere of transition metals.
Chaudret developed a method for synthesizing large aggregates (from 50 metal atoms to several tens of thousands of atoms). The method has enabled the synthesis of nanoparticles of controlled size, shape, surface and assembly of a wide variety of elements, alloys and semiconductor compounds. [3] Examples include ruthenium nanoparticles, [4] [5] iron nanocubes or cobalt nano-sticks. These particles are of interest in the fields of nanomagnetism, luminescence, electronic transport, and chemical and catalytic reactivity; it has applications in the chemo- and enantioselective labeling of molecules of biological interest and biomolecules as well as, for example, the use of magnetic and catalytic properties on the same object for the storage of renewable energies. [6]
Chaudret has been a member of the French Academy of sciences since 2005. [7] [8] and of the Academiae Europaea.
He has received numerous awards including
In 2016, he was awarded a European Research Council (ERC Advanced Grant). [11] [12]
He is a Chevalier of the Ordre of the Légion d'Honneur and of the Ordre des Palmes Académiques.
He also served as President of the Scientific Council of the CNRS (from 2010 to 2018) and President of the Scientific Council of IFPEN (from 2007 to 2011).
B. Chaudret Polyhydrides and Nanoparticles: A 35 year Trip in Organometallic Chemistry Histoire de la Recherche Contemporaine (la Revue pour l'Histoire du CNRS), Tome I, N°2, 2012, pp. 118–125
S. Sabo-Etienne, B. Chaudret, " Chemistry of bis(dihydrogen) ruthenium complexes and of their derivatives ", Coord. Chem. Rev. 1998, 178-180, 381-407
S. Sabo-Etienne, B. Chaudret, "Quantum Mechanical Exchange Coupling in polyhydride and dihydrogen complexes", Chem. Rev.1998, 98, 2077-2091.
K. Philippot, B. Chaudret, " Organometallic Approach to the Synthesis and Surface Reactivity of Noble Metal Nanoparticles", Compte-Rendus Acad Sciences 2003, 6, 1019. DOI: 10.1016/j.crci.2003.07.010
B. Chaudret, "Synthesis and Surface Reactivity of Organometallic Nanoparticles", Surface and Interfacial Organometallic Chemistry and Catalysis in Series: Topics in Organometallic Chemistry, Vol. 16 Coperet, Christope; Chaudret, Bruno (Eds.) 2005, pages 233-260.
B. Chaudret, "Organometallic approach to nanoparticles synthesis and self-organization", C.R. Physique 2005, 6, 117. DOI: 10.1016/j.crhy.2004.11.008
B. Chaudret, K. Philippot, "Organometallic nanoparticles of metals or metal oxides", Oil and Gas Science and Technology (special issue dedicated to Yves Chauvin), 2007, 62, 799. DOI: 10.2516/ogst:2007062
L.M. Martinez-Prieto, B. Chaudret Organometallic Ruthenium Nanoparticles: Synthesis, Surface Chemistry, and Insights into Ligand Coordination. Accounts of Chemical Research 2018, 51, 376.
He has published more than 420 scientific papers [13] and 20 patents.
The Suzuki reaction is an organic reaction, classified as a cross-coupling reaction, where the coupling partners are a boronic acid and an organohalide and the catalyst is a palladium(0) complex. It was first published in 1979 by Akira Suzuki, and he shared the 2010 Nobel Prize in Chemistry with Richard F. Heck and Ei-ichi Negishi for their contribution to the discovery and development of palladium-catalyzed cross-couplings in organic synthesis. This reaction is also known as the Suzuki–Miyaura reaction or simply as the Suzuki coupling. It is widely used to synthesize polyolefins, styrenes, and substituted biphenyls. Several reviews have been published describing advancements and the development of the Suzuki reaction. The general scheme for the Suzuki reaction is shown below, where a carbon-carbon single bond is formed by coupling an organoboron species (R1-BY2) with a halide (R2-X) using a palladium catalyst and a base.

In chemistry, a polyyne is any organic compound with alternating single and triple bonds; that is, a series of consecutive alkynes, (−C≡C−)
n with n greater than 1. These compounds are also called polyacetylenes, especially in the natural products and chemical ecology literature, even though this nomenclature more properly refers to acetylene polymers composed of alternating single and double bonds (−CR=CR′−). They are also sometimes referred to as oligoynes, or carbinoids after "carbyne" (−C≡C−)
∞, the hypothetical allotrope of carbon that would be the ultimate member of the series. The synthesis of this substance has been claimed several times since the 1960s, but those reports have been disputed. Indeed, the substances identified as short chains of "carbyne" in many early organic synthesis attempts would be called polyynes today.
Nanomaterial-based catalysts are usually heterogeneous catalysts broken up into metal nanoparticles in order to enhance the catalytic process. Metal nanoparticles have high surface area, which can increase catalytic activity. Nanoparticle catalysts can be easily separated and recycled. They are typically used under mild conditions to prevent decomposition of the nanoparticles.
The Willard Gibbs Award, presented by the Chicago Section of the American Chemical Society, was established in 1910 by William A. Converse (1862–1940), a former Chairman and Secretary of the Chicago Section of the society and named for Professor Josiah Willard Gibbs (1839–1903) of Yale University. Gibbs, whose formulation of the Phase Rule founded a new science, is considered by many to be the only American-born scientist whose discoveries are as fundamental in nature as those of Newton and Galileo.
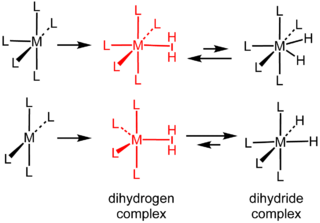
Dihydrogen complexes are coordination complexes containing intact H2 as a ligand. They are a subset of sigma complexes. The prototypical complex is W(CO)3(PCy3)2(H2). This class of compounds represent intermediates in metal-catalyzed reactions involving hydrogen. Hundreds of dihydrogen complexes have been reported. Most examples are cationic transition metals complexes with octahedral geometry.

Pierre Braunstein is a French chemist. He was director of the Laboratoire de Chimie de Coordination of Strasbourg (France) and is a member of the French Academy of Science.
A hydrogenase mimic or bio-mimetic is an enzyme mimic of hydrogenases.
Transition metal hydrides are chemical compounds containing a transition metal bonded to hydrogen. Most transition metals form hydride complexes and some are significant in various catalytic and synthetic reactions. The term "hydride" is used loosely: some so-called hydrides are acidic (e.g., H2Fe(CO)4), whereas some others are hydridic, having H−-like character (e.g., ZnH2).
David Milstein is an Israeli chemist studying homogeneous catalysis.

Organoruthenium chemistry is the chemistry of organometallic compounds containing a carbon to ruthenium chemical bond. Several organoruthenium catalysts are of commercial interest and organoruthenium compounds have been considered for cancer therapy. The chemistry has some stoichiometric similarities with organoiron chemistry, as iron is directly above ruthenium in group 8 of the periodic table. The most important reagents for the introduction of ruthenium are ruthenium(III) chloride and triruthenium dodecacarbonyl.
Guy Bertrand, born on July 17, 1952 at Limoges is a chemistry professor at the University of California, San Diego.
Anthony F. Hill is a Professor of Chemistry at the Research School of Chemistry of the Australian National University. He specializes in synthetic, organometallic and coordination chemistry. He is the author of a textbook on the subject of the organometallic chemistry of the transition metals and since 1995 has been an editor of the scientific journal/book series Advances in Organometallic Chemistry. He is a fellow of the Royal Society of Chemistry.

Didier Astruc carried out his studies in chemistry in Rennes. After a Ph. D. with professor R. Dabard in organometallic chemistry, he did post-doctoral studies with professor R. R. Schrock at the Massachusetts Institute of Technology Cambridge, Massachusetts, in the U.S. and later a sabbatical year with professor K. P. C. Vollhardt at the University of California at Berkeley. He became a CNRS Director of research in Rennes, then in 1983 full Professor of Chemistry at the University Bordeaux 1. He is known for his work on “Electron-Reservoir” complexes and dendritic molecular batteries, catalytic processes using nanoreactors and molecular recognition using gold nanoparticles and metallodendrimers. He is the author of three books, scientific publications and the editor of five books or special issues. He has been a member of the National CNRS committee from 2000 to 2008 and the President of the Coordination Chemistry Division of the Société Française de Chimie from 2000 to 2004. Didier Astruc is on the Thompson-Reuters list of the top 100 chemists who have achieved the highest citation impact scores for their chemistry papers published between 2000 and 2010. and on the list of the Highest Cited Researchers 2015 and 2016 (Thomson-Reuters). and 2017
Warren Richard Roper FRS FRSNZ FNZIC is a New Zealand chemist and Emeritus Professor at the University of Auckland.

In materials and electric battery research, cobalt oxide nanoparticles usually refers to particles of cobalt(II,III) oxide Co
3O
4 of nanometer size, with various shapes and crystal structures.
The Murai reaction is an organic reaction that uses C-H activation to create a new C-C bond between a terminal or strained internal alkene and an aromatic compound using a ruthenium catalyst. The reaction, named after Shinji Murai, was first reported in 1993. While not the first example of C-H activation, the Murai reaction is notable for its high efficiency and scope. Previous examples of such hydroarylations required more forcing conditions and narrow scope.
T. Don Tilley is a Professor of Chemistry at the University of California, Berkeley.

David Zitoun is an Israeli chemist and materials scientist.
Sophie Carenco is a researcher at the French National Center for Scientific Research, working on nanochemistry at the Laboratory of Condensed Matter Chemistry of Paris. Her research focuses on novel synthetic routes of exotic nanomaterials for energy application such as CO2 capture.
Metal-ligand cooperativity (MLC) is a mode of reactivity in which a metal and ligand of a complex are both involved in the bond breaking or bond formation of a substrate during the course of a reaction. This ligand is an actor ligand rather than a spectator, and the reaction is generally only deemed to contain MLC if the actor ligand is doing more than leaving to provide an open coordination site. MLC is also referred to as "metal-ligand bifunctional catalysis." Note that MLC is not to be confused with cooperative binding.