Related Research Articles

The cell cycle, or cell-division cycle, is the series of events that take place in a cell that causes it to divide into two daughter cells. These events include the duplication of its DNA and some of its organelles, and subsequently the partitioning of its cytoplasm, chromosomes and other components into two daughter cells in a process called cell division.

Cyclin-dependent kinases (CDKs) are a predominant group of serine/threonine protein kinases involved in the regulation of the cell cycle and its progression, ensuring the integrity and functionality of cellular machinery. These regulatory enzymes play a crucial role in the regulation of eukaryotic cell cycle and transcription, as well as DNA repair, metabolism, and epigenetic regulation, in response to several extracellular and intracellular signals. They are present in all known eukaryotes, and their regulatory function in the cell cycle has been evolutionarily conserved. The catalytic activities of CDKs are regulated by interactions with CDK inhibitors (CKIs) and regulatory subunits known as cyclins. Cyclins have no enzymatic activity themselves, but they become active once they bind to CDKs. Without cyclin, CDK is less active than in the cyclin-CDK heterodimer complex. CDKs phosphorylate proteins on serine (S) or threonine (T) residues. The specificity of CDKs for their substrates is defined by the S/T-P-X-K/R sequence, where S/T is the phosphorylation site, P is proline, X is any amino acid, and the sequence ends with lysine (K) or arginine (R). This motif ensures CDKs accurately target and modify proteins, crucial for regulating cell cycle and other functions. Deregulation of the CDK activity is linked to various pathologies, including cancer, neurodegenerative diseases, and stroke.

The restriction point (R), also known as the Start or G1/S checkpoint, is a cell cycle checkpoint in the G1 phase of the animal cell cycle at which the cell becomes "committed" to the cell cycle, and after which extracellular signals are no longer required to stimulate proliferation. The defining biochemical feature of the restriction point is the activation of G1/S- and S-phase cyclin-CDK complexes, which in turn phosphorylate proteins that initiate DNA replication, centrosome duplication, and other early cell cycle events. It is one of three main cell cycle checkpoints, the other two being the G2-M DNA damage checkpoint and the spindle checkpoint.

Cell cycle checkpoints are control mechanisms in the eukaryotic cell cycle which ensure its proper progression. Each checkpoint serves as a potential termination point along the cell cycle, during which the conditions of the cell are assessed, with progression through the various phases of the cell cycle occurring only when favorable conditions are met. There are many checkpoints in the cell cycle, but the three major ones are: the G1 checkpoint, also known as the Start or restriction checkpoint or Major Checkpoint; the G2/M checkpoint; and the metaphase-to-anaphase transition, also known as the spindle checkpoint. Progression through these checkpoints is largely determined by the activation of cyclin-dependent kinases by regulatory protein subunits called cyclins, different forms of which are produced at each stage of the cell cycle to control the specific events that occur therein.

p21Cip1, also known as cyclin-dependent kinase inhibitor 1 or CDK-interacting protein 1, is a cyclin-dependent kinase inhibitor (CKI) that is capable of inhibiting all cyclin/CDK complexes, though is primarily associated with inhibition of CDK2. p21 represents a major target of p53 activity and thus is associated with linking DNA damage to cell cycle arrest. This protein is encoded by the CDKN1A gene located on chromosome 6 (6p21.2) in humans.
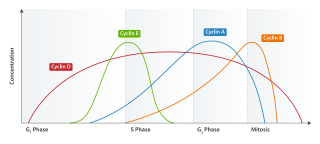
Cyclin E is a member of the cyclin family.
INK4 is a family of cyclin-dependent kinase inhibitors (CKIs). The members of this family (p16INK4a, p15INK4b, p18INK4c, p19INK4d) are inhibitors of CDK4 (hence their name INhibitors of CDK4), and of CDK6. The other family of CKIs, CIP/KIP proteins are capable of inhibiting all CDKs. Enforced expression of INK4 proteins can lead to G1 arrest by promoting redistribution of Cip/Kip proteins and blocking cyclin E-CDK2 activity. In cycling cells, there is a resassortment of Cip/Kip proteins between CDK4/5 and CDK2 as cells progress through G1. Their function, inhibiting CDK4/6, is to block progression of the cell cycle beyond the G1 restriction point. In addition, INK4 proteins play roles in cellular senescence, apoptosis and DNA repair.

Cyclin D is a member of the cyclin protein family that is involved in regulating cell cycle progression. The synthesis of cyclin D is initiated during G1 and drives the G1/S phase transition. Cyclin D protein is anywhere from 155 to 477 amino acids in length.

Cyclin-dependent kinase 2, also known as cell division protein kinase 2, or Cdk2, is an enzyme that in humans is encoded by the CDK2 gene. The protein encoded by this gene is a member of the cyclin-dependent kinase family of Ser/Thr protein kinases. This protein kinase is highly similar to the gene products of S. cerevisiae cdc28, and S. pombe cdc2, also known as Cdk1 in humans. It is a catalytic subunit of the cyclin-dependent kinase complex, whose activity is restricted to the G1-S phase of the cell cycle, where cells make proteins necessary for mitosis and replicate their DNA. This protein associates with and is regulated by the regulatory subunits of the complex including cyclin E or A. Cyclin E binds G1 phase Cdk2, which is required for the transition from G1 to S phase while binding with Cyclin A is required to progress through the S phase. Its activity is also regulated by phosphorylation. Multiple alternatively spliced variants and multiple transcription initiation sites of this gene have been reported. The role of this protein in G1-S transition has been recently questioned as cells lacking Cdk2 are reported to have no problem during this transition.

Cyclin-dependent kinase 4 also known as cell division protein kinase 4 is an enzyme that in humans is encoded by the CDK4 gene. CDK4 is a member of the cyclin-dependent kinase family.

Cell division protein kinase 6 (CDK6) is an enzyme encoded by the CDK6 gene. It is regulated by cyclins, more specifically by Cyclin D proteins and Cyclin-dependent kinase inhibitor proteins. The protein encoded by this gene is a member of the cyclin-dependent kinase, (CDK) family, which includes CDK4. CDK family members are highly similar to the gene products of Saccharomyces cerevisiae cdc28, and Schizosaccharomyces pombe cdc2, and are known to be important regulators of cell cycle progression in the point of regulation named R or restriction point.
÷
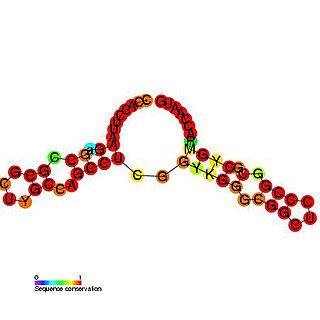
The p27 cis-regulatory element is a structured G/C rich RNA element which is involved in controlling cell cycle regulated translation of the p27kip protein in human cells.

A cyclin-dependent kinase inhibitor protein(also known as CKIs, CDIs, or CDKIs) is a protein which inhibits the enzyme cyclin-dependent kinase (CDK) and Cyclin activity by stopping the cell cycle if there are unfavorable conditions, therefore, acting as tumor suppressors. Cell cycle progression is stopped by Cyclin-dependent kinase inhibitor protein at the G1 phase. CKIs are vital proteins within the control system that point out whether the process of DNA synthesis, mitosis, and cytokines control one another. If a malfunction prevents the successful completion of DNA synthesis during the G1 phase, a signal is sent to delay or stop the progression to the S phase. Cyclin-dependent kinase inhibitor proteins are essential in the regulation of the cell cycle. If cell mutations surpass the cell cycle checkpoints during cell cycle regulation, it can result in various types of cancer.

Cyclin-dependent kinase inhibitor 1B (p27Kip1) is an enzyme inhibitor that in humans is encoded by the CDKN1B gene. It encodes a protein which belongs to the Cip/Kip family of cyclin dependent kinase (Cdk) inhibitor proteins. The encoded protein binds to and prevents the activation of cyclin E-CDK2 or cyclin D-CDK4 complexes, and thus controls the cell cycle progression at G1. It is often referred to as a cell cycle inhibitor protein because its major function is to stop or slow down the cell division cycle.

S-phase kinase-associated protein 2 is an enzyme that in humans is encoded by the SKP2 gene.

G1/S-specific cyclin-E1 is a protein that in humans is encoded by the CCNE1 gene.

Cyclin-dependent kinase inhibitor 1C , also known as CDKN1C, is a protein which in humans is encoded by the CDKN1C imprinted gene.
Cyclin-dependent kinase 4 inhibitor C is an enzyme that in humans is encoded by the CDKN2C gene.
The Neuronal cell cycle represents the life cycle of the biological cell, its creation, reproduction and eventual death. The process by which cells divide into two daughter cells is called mitosis. Once these cells are formed they enter G1, the phase in which many of the proteins needed to replicate DNA are made. After G1, the cells enter S phase during which the DNA is replicated. After S, the cell will enter G2 where the proteins required for mitosis to occur are synthesized. Unlike most cell types however, neurons are generally considered incapable of proliferating once they are differentiated, as they are in the adult nervous system. Nevertheless, it remains plausible that neurons may re-enter the cell cycle under certain circumstances. Sympathetic and cortical neurons, for example, try to reactivate the cell cycle when subjected to acute insults such as DNA damage, oxidative stress, and excitotoxicity. This process is referred to as “abortive cell cycle re-entry” because the cells usually die in the G1/S checkpoint before DNA has been replicated.
References
- 1 2 Morgan DO (2007). The Cell Cycle: Principles of Control. Primers in Biology.
- 1 2 3 Sherr CJ, Roberts JM (1999). "CDK inhibitors: positive and negative regulators of G1-phase progression". Genes & Development. 13 (12): 1501–1512. doi: 10.1101/gad.13.12.1501 . PMID 10385618.
- ↑ Gu Y, Turek CW, Morgan DO (1993). "Inhibition of CDK 2 activity in vivo by an associated 20K regulatory subunit". Nature. 366 (6456): 707–710. Bibcode:1993Natur.366..707G. doi:10.1038/366707a0. PMID 8259216. S2CID 4368793.
- ↑ Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, Beach D (1993). "p21 is a universal inhibitor of cyclin kinases". Nature. 366 (6456): 701–704. Bibcode:1993Natur.366..701X. doi:10.1038/366701a0. PMID 8259214. S2CID 4362507.
- ↑ Toyoshima H, Hunter T (1994). "p27, a novel inhibitor of G1 cyclin/cdk protein kinase activity, is related to p21". Cell. 78 (1): 67–74. doi:10.1016/0092-8674(94)90573-8. PMID 8033213. S2CID 39776582.
- ↑ Matsuoka S, Edwards M, Bai C, Parker S, Zhang P, Baldini A, Harper JW, Elledge SJ (1995). "p57kip2, a structureally distinct member of the p21 cip1 cdk inhibitor family, is a candidate tumor suppressor gene". Genes & Development. 9 (6): 650–662. doi: 10.1101/gad.9.6.650 . PMID 7729684.
- ↑ Lee MH, Reynisdottir I, Massague J (1995). "Cloning of p57kip2, a cyclin-dependent kinase inhibitor with unique domain structure and tissue distribution". Genes & Development. 9 (6): 639–649. doi: 10.1101/gad.9.6.639 . PMID 7729683.
- 1 2 Soos TJ, Kiyokawa H, Yan JS, Rubin MS, Giordano A, DeBlasio A, Bottega S, Wong B, Mendelsohn J, Koff A (1996). "Formation of p27-CDK complexes during the human mitotic cell cycle". Cell Growth & Differentiation. 7: 135–146.
- 1 2 3 LeBaer J, Garrett MD, Stevenson LF, Slingerland JM, Sandhu C, Chou HS, Fattaey A, Harlow E (1997). "New functional activities for the p21 family of CDK inhibitors". Genes & Development. 11 (7): 847–862. doi: 10.1101/gad.11.7.847 . PMID 9106657.
- ↑ Coqueret O (2003). "New roles for p21 and p27 cell-cycle inhibitors: A function for each cell compartment?". Trends in Cell Biology. 13 (2): 65–70. doi:10.1016/S0962-8924(02)00043-0. PMID 12559756.
- ↑ Nagahara H, Vocero-Akbani AM, Snyder EL, Ho A, Latham DG, Lissy NA, Becker-Hapak M, Ezhevsky SA, Dowdy SF (1998). "Transduction of full-length TAT fusion proteins into mammalian cells: TAT-p27Kip1 induces cell migration". Nature Methods. 4 (12): 1449–1452. doi:10.1038/4042. PMID 9846587. S2CID 10962704.
- 1 2 3 Besson A, Gurian-West M, Schmidt A, Hall A, Roberts JM (2004). "p27Kip1 modulates cell migration through the regulation of RhoA activation". Genes & Development. 18 (8): 851–855. doi:10.1101/gad.1185504. PMC 395846 . PMID 15078817.
- ↑ Wang YA, Elson A, Leder P (1997). "Loss of p21 increases sensitivity to ionizing radiation and delays the onset of lymphoma in atm-deficient mice". PNAS. 94 (26): 14590–14595. Bibcode:1997PNAS...9414590W. doi: 10.1073/pnas.94.26.14590 . PMC 25064 . PMID 9405657.
- ↑ Russo AA, Jeffrey PD, Patten AK, Massague J, Pavletich NP (1996). "Crystal structure of the p27Kip1 cyclin-dependent kinase inhibitor bound to the cyclin A-cdk2 complex". Nature. 382 (6589): 325–331. Bibcode:1996Natur.382..325R. doi:10.1038/382325a0. PMID 8684460. S2CID 4284942.
- ↑ Cheng M, Olivier P, Diehl JA, Fero M, Roussel MF, Roberts JM, Sherr CJ (1999). "The p21Cip1 and p27Kip1 CDK 'inhibitors' are essential activators of cyclin D-dependent kinases in murine fibroblasts". EMBO Journal. 18 (6): 1571–1583. doi:10.1093/emboj/18.6.1571. PMC 1171245 . PMID 10075928.
- ↑ Jiang H, Chou HS, Zhu L (1998). "Requirement of cyclin E-cdk2 inhibition in p16INK4a-mediated growth suppression". Molecular and Cellular Biology. 18 (9): 5284–5290. doi:10.1128/MCB.18.9.5284. PMC 109114 . PMID 9710613.
- ↑ Levkau B, Koyama H, Raines EW, Clurman BE, Herren B, Orth K, Roberts JM, Ross R (1998). "Cleavage of p21Cip?Waf1 and P27Kip1 mediates apoptosis in endothelial cells through activation of Cdk2: role of a caspase cascade". Molecular Cell. 1 (4): 553–563. doi: 10.1016/S1097-2765(00)80055-6 . PMID 9660939.
- ↑ Yan Y, Frisen J, Lee MH, Massague J, Barbacid M (1997). "Ablation of the CDK inhibitor p57Kip2 results in increased apoptosis and delayed differentiation during mouse development". Genes & Development. 11 (8): 973–983. doi: 10.1101/gad.11.8.973 . PMID 9136926.
- ↑ Chang TS, Kim MJ, Ryoo K, Park J, Eom SJ, Shim J, Nakayama KI, Nakayama K, Tomita M, Takahashi K, et al. (2003). "p57Kip2 modulates stress-activated signaling by inhibiting c-Jun NH2-terminal kinase/stress-activated protein kinase". Journal of Biological Chemistry. 278 (48): 1158–1164. doi: 10.1074/jbc.M309421200 . PMID 12963725.
- ↑ Nguyen L, Besson A, Heng J, Schurrmans C, Teboul L, Philpott A, Roberts JM, Guillemot F (2006). "P27Kip1 independently promotes neuronal differentiation and migration in the cerebral cortex". Genes & Development. 20 (11): 1511–1524. doi:10.1101/gad.377106. PMC 1475763 . PMID 16705040.
- ↑ McAllister SS, Becker-Hapak M, Pintucci G, Pagano M, Dowdy SF (2003). "Novel p27(kip1) C-terminal scatter domain mediates Rac-dependent cell migration independent of cell cycle arrest functions". Molecular and Cellular Biology. 23 (1): 216–228. doi:10.1128/MCB.23.1.216-228.2003. PMC 140659 . PMID 12482975.
- ↑ Catzavelos C, Bhattacharya N, Ung YC, Wilson JA, Roncari L, Sandhu C, Shaw P, Yeger H, Morava-Protzner I, Kapusta L, Franssen E, Pritchard KI, Slingerland JM (1997). "Decreased levels of the cell-cycle inhibitor p27Kip1 protein: Prognostic implications in primary breast cancer". Nature Medicine. 3 (2): 227–230. doi:10.1038/nm0297-227. PMID 9018244. S2CID 25460889.
- ↑ Loda M, Cukor B, Tam SW, Lavin P, Fiorentino M, Draetta GF, Jessup JM, Pagano M (1997). "Increased proteasome-dependent degradation of the cyclin-dependent kinase inhibitor p27 in aggressive colorectal carcinomas". Nature Medicine. 3 (2): 231–234. doi:10.1038/nm0297-231. PMID 9018245. S2CID 3164478.
- ↑ Nakayama K, Ishida N, Shirane M, Inomata A, Inoue T, Shishido N, Horii I, Loh DY, Nakayama K (1996). "Mice lacking p27Kip1 display increased body size, multiple organ hyperplasia, retinal dysplasia and pituitary tumors". Cell. 85 (5): 701–720. doi: 10.1016/S0092-8674(00)81237-4 . PMID 8646779. S2CID 2009281.
- ↑ Fero ML, Rivkin M, Tasch M, Porter P, Carow CE, Firpo E, Poyak K, Tsai LH, Broudy V, Perlmutter RM, et al. (1996). "A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27(Kip1)-deficient mice". Cell. 85 (5): 733–744. doi: 10.1016/S0092-8674(00)81239-8 . PMID 8646781. S2CID 15490866.
- ↑ Fero ML, Randel E, Gurley KE, Roberts JM, Kemp CJ (1998). "The murine gene p27Kip1 is haplo-insufficient for tumour suppression". Nature. 396 (6707): 177–180. Bibcode:1998Natur.396..177F. doi:10.1038/24179. PMC 5395202 . PMID 9823898.
- 1 2 Besson A, Assoian RK, Roberts JM (2004). "Regulation of the cytoskeleton: an oncogenic function for CDK inhibitors?". Nature Reviews Cancer. 4 (12): 948–955. doi:10.1038/nrc1501. PMID 15573116. S2CID 12663308.
- ↑ Blain SW, Scher HI, Cordon-Cardo C, Koff A (2003). "p27 as a target for cancer therapeutics". Cancer Cell. 3 (2): 111–115. doi: 10.1016/S1535-6108(03)00026-6 . PMID 12620406.
- ↑ Denicourt C, Saenz CC, Datnow B, Cui XS, Dowdy SF (2007). "Relocalized p27Kip1 tumor suppressor functions as a cytoplasmic metastatic oncogene in melanoma". Cancer Research. 67 (19): 9238–9243. doi: 10.1158/0008-5472.CAN-07-1375 . PMID 17909030.
- ↑ Slingerland J, Pagano MA (2000). "Regulation of the CDK inhibitor p27 and its deregulation in cancer". Journal of Cellular Physiology. 183 (1): 10–17. doi:10.1002/(SICI)1097-4652(200004)183:1<10::AID-JCP2>3.0.CO;2-I. PMID 10699961. S2CID 8756686.