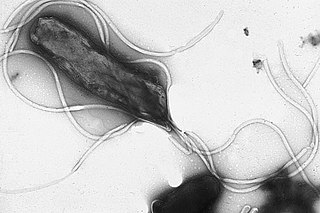
Helicobacter pylori, previously known as Campylobacter pylori, is a gram-negative, microaerophilic, spiral (helical) bacterium usually found in the stomach. Its helical shape is thought to have evolved in order to penetrate the mucoid lining of the stomach and thereby establish infection. The bacterium was first identified in 1982 by Australian doctors Barry Marshall and Robin Warren. H. pylori has been associated with the mucosa-associated lymphoid tissue in the stomach, esophagus, colon, rectum, or tissues around the eye, and of lymphoid tissue in the stomach.

Helicobacter is a genus of Gram-negative bacteria possessing a characteristic helical shape. They were initially considered to be members of the genus Campylobacter, but in 1989, Goodwin et al. published sufficient reasons to justify the new genus name Helicobacter. The genus Helicobacter contains about 35 species.
Secretion is the movement of material from one point to another, such as a secreted chemical substance from a cell or gland. In contrast, excretion, is the removal of certain substances or waste products from a cell or organism. The classical mechanism of cell secretion is via secretory portals at the cell plasma membrane called porosomes. Porosomes are permanent cup-shaped lipoprotein structure at the cell plasma membrane, where secretory vesicles transiently dock and fuse to release intra-vesicular contents from the cell.

Achlorhydria and hypochlorhydria refer to states where the production of hydrochloric acid in gastric secretions of the stomach and other digestive organs is absent or low, respectively. It is associated with various other medical problems.
MALT lymphoma (MALToma) is a form of lymphoma involving the mucosa-associated lymphoid tissue (MALT), frequently of the stomach, but virtually any mucosal site can be afflicted. It is a cancer originating from B cells in the marginal zone of the MALT, and is also called extranodal marginal zone B cell lymphoma.

Martin J. Blaser is the director of the Center for Advanced Biotechnology and Medicine (CABM) at Rutgers (NJ) Biomedical and Health Sciences (RBHS) and the Henry Rutgers Chair of the Human Microbiome and professor of medicine and microbiology at the Rutgers Robert Wood Johnson Medical School in New Jersey.

NlaIII is a type II restriction enzyme isolated from Neisseria lactamica. As part of the restriction modification system, NlaIII is able to prevent foreign DNA from integrating into the host genome by cutting double stranded DNA into fragments at specific sequences. This results in further degradation of the fragmented foreign DNA and prevents it from infecting the host genome.

Tyrosine-protein phosphatase non-receptor type 11 (PTPN11) also known as protein-tyrosine phosphatase 1D (PTP-1D), Src homology region 2 domain-containing phosphatase-2 (SHP-2), or protein-tyrosine phosphatase 2C (PTP-2C) is an enzyme that in humans is encoded by the PTPN11 gene. PTPN11 is a protein tyrosine phosphatase (PTP) Shp2.
This is a timeline of the events relating to the discovery that peptic ulcer disease and some cancers are caused by H. pylori. In 2005, Barry Marshall and Robin Warren were awarded the Nobel Prize in Physiology or Medicine for their discovery that peptic ulcer disease (PUD) was primarily caused by Helicobacter pylori, a bacterium with affinity for acidic environments, such as the stomach. As a result, PUD that is associated with H. pylori is currently treated with antibiotics used to eradicate the infection. For decades prior to their discovery, it was widely believed that PUD was caused by excess acid in the stomach. During this time, acid control was the primary method of treatment for PUD, to only partial success. Among other effects, it is now known that acid suppression alters the stomach milieu to make it less amenable to H. pylori infection.
Enolase 1 (ENO1), more commonly known as alpha-enolase, is a glycolytic enzyme expressed in most tissues, one of the isozymes of enolase. Each isoenzyme is a homodimer composed of 2 alpha, 2 gamma, or 2 beta subunits, and functions as a glycolytic enzyme. Alpha-enolase, in addition, functions as a structural lens protein (tau-crystallin) in the monomeric form. Alternative splicing of this gene results in a shorter isoform that has been shown to bind to the c-myc promoter and function as a tumor suppressor. Several pseudogenes have been identified, including one on the long arm of chromosome 1. Alpha-enolase has also been identified as an autoantigen in Hashimoto encephalopathy.

Barringtonia acutangula is a species of Barringtonia native to coastal wetlands in southern Asia and northern Australasia, from Afghanistan east to the Philippines and Queensland. Common names include freshwater mangrove, itchytree and mango-pine.

Cancer bacteria are bacteria infectious organisms that are known or suspected to cause cancer. While cancer-associated bacteria have long been considered to be opportunistic, there is some evidence that bacteria may be directly carcinogenic. The strongest evidence to date involves the bacterium H. pylori and its role in gastric cancer.

Histidine kinases (HK) are multifunctional, and in non-animal kingdoms, typically transmembrane, proteins of the transferase class of enzymes that play a role in signal transduction across the cellular membrane. The vast majority of HKs are homodimers that exhibit autokinase, phosphotransfer, and phosphatase activity. HKs can act as cellular receptors for signaling molecules in a way analogous to tyrosine kinase receptors (RTK). Multifunctional receptor molecules such as HKs and RTKs typically have portions on the outside of the cell that bind to hormone- or growth factor-like molecules, portions that span the cell membrane, and portions within the cell that contain the enzymatic activity. In addition to kinase activity, the intracellular domains typically have regions that bind to a secondary effector molecule or complex of molecules that further propagate signal transduction within the cell. Distinct from other classes of protein kinases, HKs are usually parts of a two-component signal transduction mechanisms in which HK transfers a phosphate group from ATP to a histidine residue within the kinase, and then to an aspartate residue on the receiver domain of a response regulator protein. More recently, the widespread existence of protein histidine phosphorylation distinct from that of two-component histidine kinases has been recognised in human cells. In marked contrast to Ser, Thr and Tyr phosphorylation, the analysis of phosphorylated Histidine using standard biochemical and mass spectrometric approaches is much more challenging, and special procedures and separation techniques are required for their preservation alongside classical Ser, Thr and Tyr phosphorylation on proteins isolated from human cells.

NLRX1 or NLR family member X1, short for nucleotide-binding oligomerization domain, leucine rich repeat containing X1 is a protein that in humans is encoded by the NLRX1 gene. It is also known as NOD-like receptor X1, NLR family, X1, NOD5, NOD9, and CLR11.3, and is a member of the NOD-like receptor family of pattern recognition receptors.
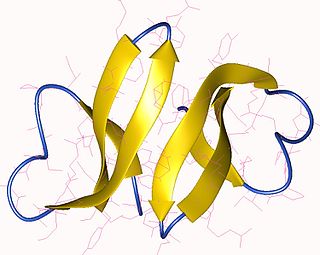
Defensin, alpha 3 (DEFA3) also known as human alpha defensin 3, human neutrophil peptide 3 (HNP-3) or neutrophil defensin 3 is a human protein that is encoded by the DEFA3 gene. Human alpha defensin 3 belongs to the alpha defensin family of antimicrobial peptides.

Estimates place the worldwide risk of cancers from infectious causes at 16.1%. Viral infections are risk factors for cervical cancer, 80% of liver cancers, and 15–20% of the other cancers. This proportion varies in different regions of the world from a high of 32.7% in Sub-Saharan Africa to 3.3% in Australia and New Zealand. Helicobacter pylori is associated with stomach cancer, and Mycobacterium, some other bacteria and parasites also have an effect.

In molecular biology, the catalase-related immune-responsive domain is a protein domain found in catalases. This domain carries the immune-responsive amphipathic octa-peptide that is recognised by T cells.
Helicobacter pylori virulence factor CagA is a 120–145kDa protein encoded on the 40kb cag pathogenicity island (PAI). H. pylori strains can be divided into CagA positive or negative strains. Approximately 60% of H. pylori strains isolated in Western countries carry cag PAI, whereas almost all of the East Asian isolates are cag PAI-positive

Mark Achtman FRS is Professor of Bacterial Population Genetics at Warwick Medical School, part of the University of Warwick in the UK.
Proteins currently known to belong to the Ni2+-Co2+ Transporter (NiCoT) family (TC# 2.A.52) can be found in organisms ranging from Gram-negative and Gram-positive bacteria to archaea and some eukaryotes. Members of this family catalyze uptake of Ni2+ and/or Co2+ in a proton motive force-dependent process.