
Carbon dioxide is a chemical compound with the chemical formula CO2. It is made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature, and as the source of available carbon in the carbon cycle, atmospheric CO2 is the primary carbon source for life on Earth. In the air, carbon dioxide is transparent to visible light but absorbs infrared radiation, acting as a greenhouse gas. Carbon dioxide is soluble in water and is found in groundwater, lakes, ice caps, and seawater. When carbon dioxide dissolves in water, it forms carbonate and mainly bicarbonate, which causes ocean acidification as atmospheric CO2 levels increase.

Gas chromatography (GC) is a common type of chromatography used in analytical chemistry for separating and analyzing compounds that can be vaporized without decomposition. Typical uses of GC include testing the purity of a particular substance, or separating the different components of a mixture. In preparative chromatography, GC can be used to prepare pure compounds from a mixture.
Calcination is thermal treatment of a solid chemical compound (e.g. mixed carbonate ores) whereby the compound is raised to high temperature without melting under restricted supply of ambient oxygen (i.e. gaseous O2 fraction of air), generally for the purpose of removing impurities or volatile substances and/or to incur thermal decomposition.

Hopcalite is the trade name for a number of mixtures that mainly consist of oxides of copper and manganese, which are used as catalysts for the conversion of carbon monoxide to carbon dioxide when exposed to the oxygen in the air at room temperature.

A fossil fuel power station is a thermal power station which burns a fossil fuel, such as coal, oil, or natural gas, to produce electricity. Fossil fuel power stations have machinery to convert the heat energy of combustion into mechanical energy, which then operates an electrical generator. The prime mover may be a steam turbine, a gas turbine or, in small plants, a reciprocating gas engine. All plants use the energy extracted from the expansion of a hot gas, either steam or combustion gases. Although different energy conversion methods exist, all thermal power station conversion methods have their efficiency limited by the Carnot efficiency and therefore produce waste heat.
Pyrometallurgy is a branch of extractive metallurgy. It consists of the thermal treatment of minerals and metallurgical ores and concentrates to bring about physical and chemical transformations in the materials to enable recovery of valuable metals. Pyrometallurgical treatment may produce products able to be sold such as pure metals, or intermediate compounds or alloys, suitable as feed for further processing. Examples of elements extracted by pyrometallurgical processes include the oxides of less reactive elements like iron, copper, zinc, chromium, tin, and manganese.
A coolant is a substance, typically liquid, that is used to reduce or regulate the temperature of a system. An ideal coolant has high thermal capacity, low viscosity, is low-cost, non-toxic, chemically inert and neither causes nor promotes corrosion of the cooling system. Some applications also require the coolant to be an electrical insulator.

Total organic carbon (TOC) is an analytical parameter representing the concentration of organic carbon in a sample. TOC determinations are made in a variety of application areas. For example, TOC may be used as a non-specific indicator of water quality, or TOC of source rock may be used as one factor in evaluating a petroleum play. For marine surface sediments average TOC content is 0.5% in the deep ocean, and 2% along the eastern margins.
Combustion analysis is a method used in both organic chemistry and analytical chemistry to determine the elemental composition of a pure organic compound by combusting the sample under conditions where the resulting combustion products can be quantitatively analyzed. Once the number of moles of each combustion product has been determined the empirical formula or a partial empirical formula of the original compound can be calculated.
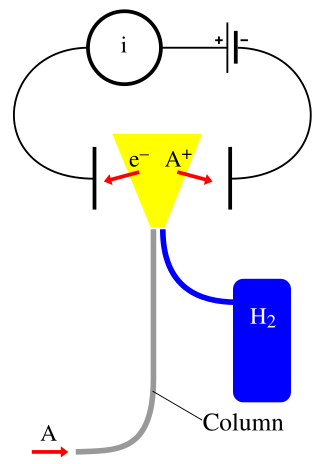
A flame ionization detector (FID) is a scientific instrument that measures analytes in a gas stream. It is frequently used as a detector in gas chromatography. The measurement of ions per unit time makes this a mass sensitive instrument. Standalone FIDs can also be used in applications such as landfill gas monitoring, fugitive emissions monitoring and internal combustion engine emissions measurement in stationary or portable instruments.
A carbon dioxide sensor or CO2 sensor is an instrument for the measurement of carbon dioxide gas. The most common principles for CO2 sensors are infrared gas sensors (NDIR) and chemical gas sensors. Measuring carbon dioxide is important in monitoring indoor air quality, the function of the lungs in the form of a capnograph device, and many industrial processes.

A nondispersive infrared sensor is a simple spectroscopic sensor often used as a gas detector. It is non-dispersive in the fact that no dispersive element is used to separate out the broadband light into a narrow spectrum suitable for gas sensing. The majority of NDIR sensors use a broadband lamp source and an optical filter to select a narrow band spectral region that overlaps with the absorption region of the gas of interest. In this context narrow may be 50-300nm bandwidth. Modern NDIR sensors may use Microelectromechanical systems (MEMs) or mid IR LED sources, with or without an optical filter.

A carbon dioxide scrubber is a piece of equipment that absorbs carbon dioxide (CO2). It is used to treat exhaust gases from industrial plants or from exhaled air in life support systems such as rebreathers or in spacecraft, submersible craft or airtight chambers. Carbon dioxide scrubbers are also used in controlled atmosphere (CA) storage and carbon capture and storage processes.
A gas detector is a device that detects the presence of gases in an area, often as part of a safety system. A gas detector can sound an alarm to operators in the area where the leak is occurring, giving them the opportunity to leave. This type of device is important because there are many gases that can be harmful to organic life, such as humans or animals.
The thermal conductivity detector (TCD), also known as a katharometer, is a bulk property detector and a chemical specific detector commonly used in gas chromatography. This detector senses changes in the thermal conductivity of the column eluent and compares it to a reference flow of carrier gas. Since most compounds have a thermal conductivity much less than that of the common carrier gases of helium or hydrogen, when an analyte elutes from the column the effluent thermal conductivity is reduced, and a detectable signal is produced.
The Glossary of fuel cell terms lists the definitions of many terms used within the fuel cell industry. The terms in this fuel cell glossary may be used by fuel cell industry associations, in education material and fuel cell codes and standards to name but a few.
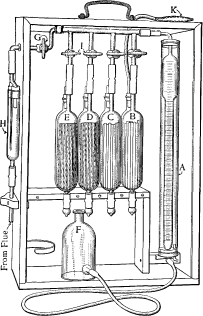
An Orsat gas analyser or Orsat apparatus is a piece of laboratory equipment used to analyse a gas sample for its oxygen, carbon monoxide and carbon dioxide content. Although largely replaced by instrumental techniques, the Orsat remains a reliable method of measurement and is relatively simple to use.

Greenhouse gas monitoring is the direct measurement of greenhouse gas emissions and levels. There are several different methods of measuring carbon dioxide concentrations in the atmosphere, including infrared analyzing and manometry. Methane and nitrous oxide are measured by other instruments. Greenhouse gases are measured from space such as by the Orbiting Carbon Observatory and networks of ground stations such as the Integrated Carbon Observation System.
In analytical chemistry, the Dumas method is a method of elemental analysis for the quantitative determination of nitrogen in chemical substances based on a method first described by Jean-Baptiste Dumas in 1826.

An exhaust gas analyser or exhaust carbon monoxide (CO) analyser is an instrument for the measurement of carbon monoxide among other gases in the exhaust, caused by an incorrect combustion, the Lambda coefficient measurement is the most common.










