A rosé is a type of wine that incorporates some of the color from the grape skins, but not enough to qualify it as a red wine. It may be the oldest known type of wine, as it is the most straightforward to make with the skin contact method. The pink color can range from a pale "onionskin" orange to a vivid near-purple, depending on the grape varieties used and winemaking techniques. Usually, the wine is labelled rosé in French, Portuguese, and English-speaking countries, rosado in Spanish, or rosato in Italian.
A wine fault is a sensory-associated (organoleptic) characteristic of a wine that is unpleasant, and may include elements of taste, smell, or appearance, elements that may arise from a "chemical or a microbial origin", where particular sensory experiences might arise from more than one wine fault. Wine faults may result from poor winemaking practices or storage conditions that lead to wine spoilage.

The following outline is provided as an overview of and topical guide to wine:

The phenolic content in wine refers to the phenolic compounds—natural phenol and polyphenols—in wine, which include a large group of several hundred chemical compounds that affect the taste, color and mouthfeel of wine. These compounds include phenolic acids, stilbenoids, flavonols, dihydroflavonols, anthocyanins, flavanol monomers (catechins) and flavanol polymers (proanthocyanidins). This large group of natural phenols can be broadly separated into two categories, flavonoids and non-flavonoids. Flavonoids include the anthocyanins and tannins which contribute to the color and mouthfeel of the wine. The non-flavonoids include the stilbenoids such as resveratrol and phenolic acids such as benzoic, caffeic and cinnamic acids.

Colorino is a red Italian wine grape variety planted primarily in Tuscany. The grape is known for its deep dark colouring and is used primarily as a colouring agent in red blends. In the history of Chianti it played a minor role, mostly for its affinity and use to the governo winemaking technique. Like Canaiolo, Colorino did not rot easily while going through the partial drying process to later be added to the fermenting grape must. However, the grape did not provide the same level of fruit and softening effect that Canaiolo did and fell out of favour. In the late 1980s, there was a surge of interest in the variety among Tuscan winemakers who saw in this local grape variety similarity to the role Petit Verdot plays in Bordeaux blends. Colorino was planted and used to add darker colours and structure from phenolic compounds in the grape's thick skin without the overpowering aromatics that Cabernet Sauvignon could add. This enthusiasm was short-lived and by the turn of the 21st century Colorino returned once again to a minor role in Tuscan wines.

The aging of wine is potentially able to improve the quality of wine. This distinguishes wine from most other consumable goods. While wine is perishable and capable of deteriorating, complex chemical reactions involving a wine's sugars, acids and phenolic compounds can alter the aroma, color, mouthfeel and taste of the wine in a way that may be more pleasing to the taster. The ability of a wine to age is influenced by many factors including grape variety, vintage, viticultural practices, wine region and winemaking style. The condition that the wine is kept in after bottling can also influence how well a wine ages and may require significant time and financial investment. The quality of an aged wine varies significantly bottle-by-bottle, depending on the conditions under which it was stored, and the condition of the bottle and cork, and thus it is said that rather than good old vintages, there are good old bottles. There is a significant mystique around the aging of wine, as its chemistry was not understood for a long time, and old wines are often sold for extraordinary prices. However, the vast majority of wine is not aged, and even wine that is aged is rarely aged for long; it is estimated that 90% of wine is meant to be consumed within a year of production, and 99% of wine within 5 years.
This glossary of winemaking terms lists some of terms and definitions involved in making wine, fruit wine, and mead.

In winemaking, clarification and stabilization are the processes by which insoluble matter suspended in the wine is removed before bottling. This matter may include dead yeast cells (lees), bacteria, tartrates, proteins, pectins, various tannins and other phenolic compounds, as well as pieces of grape skin, pulp, stems and gums. Clarification and stabilization may involve fining, filtration, centrifugation, flotation, refrigeration, pasteurization, and/or barrel maturation and racking.

Grandinin is an ellagitannin. It can be found in Melaleuca quinquenervia leaves and in oaks species like the North American white oak and European red oak. It shows antioxydant activity. It is an astringent compound. It is also found in wine, red or white, aged in oak barrels.
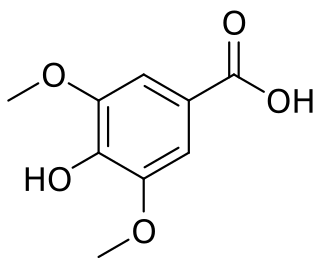
Syringic acid is a naturally occurring phenolic compound and dimethoxybenzene that is commonly found as a plant metabolite.

Acutissimin A is a flavono-ellagitannin, a type of tannin formed from the linking of a flavonoid with an ellagitannin.

In biochemistry, naturally occurring phenols are natural products containing at least one phenol functional group. Phenolic compounds are produced by plants and microorganisms. Organisms sometimes synthesize phenolic compounds in response to ecological pressures such as pathogen and insect attack, UV radiation and wounding. As they are present in food consumed in human diets and in plants used in traditional medicine of several cultures, their role in human health and disease is a subject of research. Some phenols are germicidal and are used in formulating disinfectants.

The grape reaction product is a phenolic compound explaining the disappearance of caftaric acid from grape must during processing. It is also found in aged red wines. Its enzymatic production by polyphenol oxidase is important in limiting the browning of musts, especially in white wine production. The product can be recreated in model solutions.

Astringin is a stilbenoid, the 3-β-D-glucoside of piceatannol. It can be found in the bark of Picea sitchensis and Picea abies.

Vitisin A is a natural phenol found in red wines. It is a pyranoanthocyanin.

Vitisin B is a natural phenol found in red wines. It is a pyranoanthocyanin.
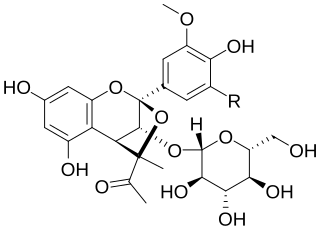
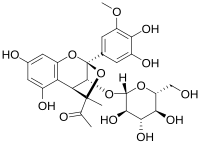
Castavinols are natural phenolic compounds found in red wines. These molecules are colorless and are derived from anthocyanin pigments. Thus their formation leads to a wine color loss.
4-Vinylphenol is an organic compound with the formula C2H3C6H4OH. It is the most studied of the three isomeric vinylphenols. It is a white volatile solid.

Delphinidin 3-O-(6-p-coumaroyl)glucoside is a p-coumaroylated anthocyanin. It can be found in some red Vitis vinifera grape cultivars and in red wine.
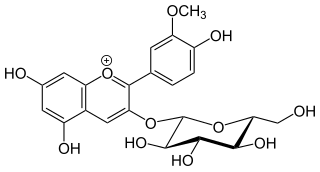
Peonidin-3-O-glucoside is anthocyanin. It is found in fruits and berries, in red Vitis vinifera grapes and red wine, in red onions and in purple corn. It is dark red to purple in colour.