
An electrochemical cell is a device that generates electrical energy from chemical reactions. Electrical energy can also be applied to these cells to cause chemical reactions to occur. Electrochemical cells that generate an electric current are called voltaic or galvanic cells and those that generate chemical reactions, via electrolysis for example, are called electrolytic cells.

In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from naturally occurring sources such as ores using an electrolytic cell. The voltage that is needed for electrolysis to occur is called the decomposition potential. The word "lysis" means to separate or break, so in terms, electrolysis would mean "breakdown via electricity."
In electrochemistry, the electrochemical potential (ECP), μ, is a thermodynamic measure of chemical potential that does not omit the energy contribution of electrostatics. Electrochemical potential is expressed in the unit of J/mol.
The chloralkali process is an industrial process for the electrolysis of sodium chloride (NaCl) solutions. It is the technology used to produce chlorine and sodium hydroxide, which are commodity chemicals required by industry. Thirty five million tons of chlorine were prepared by this process in 1987. In 2022, this had increased to about 83 million tonnes. The chlorine and sodium hydroxide produced in this process are widely used in the chemical industry.

Nafion is a brand name for a sulfonated tetrafluoroethylene based fluoropolymer-copolymer synthesized in 1962 by Dr. Donald J. Connolly at the DuPont Experimental Station in Wilmington Delaware. Additional work on the polymer family was performed in the late 1960s by Dr. Walther Grot of DuPont. Nafion is a brand of the Chemours company. It is the first of a class of synthetic polymers with ionic properties that are called ionomers. Nafion's unique ionic properties are a result of incorporating perfluorovinyl ether groups terminated with sulfonate groups onto a tetrafluoroethylene (PTFE) backbone. Nafion has received a considerable amount of attention as a proton conductor for proton exchange membrane (PEM) fuel cells because of its excellent chemical and mechanical stability in the harsh conditions of this application.
A proton-exchange membrane, or polymer-electrolyte membrane (PEM), is a semipermeable membrane generally made from ionomers and designed to conduct protons while acting as an electronic insulator and reactant barrier, e.g. to oxygen and hydrogen gas. This is their essential function when incorporated into a membrane electrode assembly (MEA) of a proton-exchange membrane fuel cell or of a proton-exchange membrane electrolyser: separation of reactants and transport of protons while blocking a direct electronic pathway through the membrane.
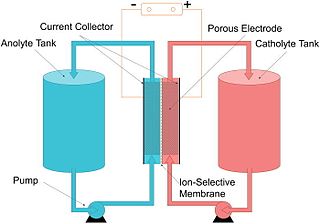
A flow battery, or redox flow battery, is a type of electrochemical cell where chemical energy is provided by two chemical components dissolved in liquids that are pumped through the system on separate sides of a membrane. Ion transfer inside the cell occurs across the membrane while the liquids circulate in their respective spaces.
The Castner–Kellner process is a method of electrolysis on an aqueous alkali chloride solution to produce the corresponding alkali hydroxide, invented by American Hamilton Castner and Austrian Carl Kellner in the 1890s. It is a type of chloralkali process, but in this role it is gradually being replaced by membrane electrolysis which has lower energy cost and fewer environmental concerns.
Gas diffusion electrodes (GDE) are electrodes with a conjunction of a solid, liquid and gaseous interface, and an electrical conducting catalyst supporting an electrochemical reaction between the liquid and the gaseous phase.
Chlorine gas can be produced by extracting from natural materials, including the electrolysis of a sodium chloride solution (brine) and other ways.
Electrochemical engineering is the branch of chemical engineering dealing with the technological applications of electrochemical phenomena, such as electrosynthesis of chemicals, electrowinning and refining of metals, flow batteries and fuel cells, surface modification by electrodeposition, electrochemical separations and corrosion.
Richard Yeo Swee Chye is an American scientist with 17 U.S. patents, best known for his research on disposable diapers.
Yang Shao-Horn is a Chinese American scholar, Professor of Mechanical Engineering and Materials Science and Engineering and a member of Research Laboratory of Electronics at the Massachusetts Institute of Technology. She is known for research on understanding and controlling of processes for storing electrons in chemical bonds towards zero-carbon energy and chemicals.
Maria Skyllas-Kazacos is an Australian chemical engineer best known for her pioneering work of the vanadium redox battery, which she created at the University of New South Wales in the 1980s. Her design used sulfuric acid electrolytes and was patented by the university. In 1999 she was appointed a Member of the Order of Australia "for service to science and technology, particularly in the development of the vanadium redox battery as an alternative power source".
Shelley D. Minteer is an American academic and chemistry professor at the University of Utah. Minteer field of study focuses on the interface between biocatalysts and enzyme-based electrodes for biofuel cells and sensors.
Viola Ingrid Birss is a Professor of Chemistry at the University of Calgary. She works on electrochemistry and the development of nanomaterials for sustainable energy and sensing applications. She has demonstrated a metal oxide perovskite that can be used as the air and fuel electrode in solid oxide fuel cells, as well as creating nanoporous carbon scaffolds to be used in batteries and capacitors.
Héctor Daniel Abruña is a Puerto Rican physical chemist whose work focuses on electrochemistry, molecular electronics, fuel cells, batteries, and electrocatalysis. Abruña is director of the Energy Materials Center and Emile M. Chamot professor for chemistry at Cornell University. He became a Fellow of the American Association for the Advancement of Science in 2006, a Member of the American Academy of Arts and Sciences in 2007, and a Member of the National Academy of Sciences in 2018. Abruña conducts research into battery and fuel cell systems using electrochemical techniques and X-ray microscopy and spectroscopy methods.

Bruno Georges Pollet BSc(Hons) MSc PhD FIAHE FRSC, is an electrochemist and electrochemical engineer, a Fellow of the Royal Society of Chemistry, a Fellow of the International Association for Hydrogen Energy, a full professor of chemistry, director of the Green Hydrogen Lab and member of the Hydrogen Research Institute at the Université du Québec à Trois-Rivières in Canada. He has worked on Hydrogen Energy in the UK, Japan, South Africa, Norway and Canada, and has both industrial and academic experience. He is a prolific scholar, collaborator, and mentor. He is also regarded as one of the most prominent Hydrogen experts and one of the Hydrogen "influencers" in the world.
Peter Strasser is a German chemist. He is the winner of the 2021 Faraday Medal.

Kenneth Ikechukwu Ozoemena is a Nigerian physical chemist, materials scientist, and academic. He is a research professor at the University of the Witwatersrand (Wits) in Johannesburg where he Heads the South African SARChI Chair in Materials Electrochemistry and Energy Technologies (MEET), supported by the Department of Science and Innovation (DSI), National Research Foundation (NRF) and Wits.






