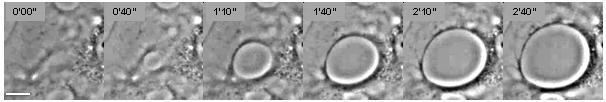
Cellular dewetting refers to the process of nucleation and enlargement of transendothelial cell macroaperture (TEM) tunnels in endothelial cells (Figure 1). [1] This phenomenon is analogous to the nucleation and growth of dry patches in viscous liquids spreading on a non-wettable substrate (Figure 2). [2] Cellular dewetting is triggered by several protein toxins from pathogenic bacteria, notably the EDIN-like factors from Staphylococcus aureus and from Clostridium botulinum, as well as edema toxin from Bacillus anthracis. [3] [4] TEMs form in response to the rupture of cytoskeleton physical connections through the cytoplasm due to inhibition of the RhoA/ROCK pathway or to induction of the flux of cyclic-AMP (cAMP) broad signaling molecule. [4] [5]

