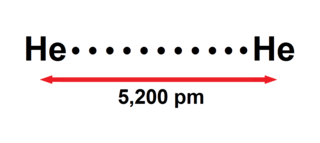Ionization is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons, often in conjunction with other chemical changes. The resulting electrically charged atom or molecule is called an ion. Ionization can result from the loss of an electron after collisions with subatomic particles, collisions with other atoms, molecules, electrons, positrons, protons, antiprotons and ions, or through the interaction with electromagnetic radiation. Heterolytic bond cleavage and heterolytic substitution reactions can result in the formation of ion pairs. Ionization can occur through radioactive decay by the internal conversion process, in which an excited nucleus transfers its energy to one of the inner-shell electrons causing it to be ejected.
The electron affinity (Eea) of an atom or molecule is defined as the amount of energy released when an electron attaches to a neutral atom or molecule in the gaseous state to form an anion.

Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a mass spectrum, a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is used in many different fields and is applied to pure samples as well as complex mixtures.

An ion source is a device that creates atomic and molecular ions. Ion sources are used to form ions for mass spectrometers, optical emission spectrometers, particle accelerators, ion implanters and ion engines.

Electron ionization is an ionization method in which energetic electrons interact with solid or gas phase atoms or molecules to produce ions. EI was one of the first ionization techniques developed for mass spectrometry. However, this method is still a popular ionization technique. This technique is considered a hard ionization method, since it uses highly energetic electrons to produce ions. This leads to extensive fragmentation, which can be helpful for structure determination of unknown compounds. EI is the most useful for organic compounds which have a molecular weight below 600. Also, several other thermally stable and volatile compounds in solid, liquid and gas states can be detected with the use of this technique when coupled with various separation methods.

Tandem mass spectrometry, also known as MS/MS or MS2, is a technique in instrumental analysis where two or more stages of analysis using one or more mass analyzer are performed with an additional reaction step in between these analyses to increase their abilities to analyse chemical samples. A common use of tandem MS is the analysis of biomolecules, such as proteins and peptides.

Chemical ionization (CI) is a soft ionization technique used in mass spectrometry. This was first introduced by Burnaby Munson and Frank H. Field in 1966. This technique is a branch of gaseous ion-molecule chemistry. Reagent gas molecules are ionized by electron ionization to form reagent ions, which subsequently react with analyte molecules in the gas phase to create analyte ions for analysis by mass spectrometry. Negative chemical ionization (NCI), charge-exchange chemical ionization, atmospheric-pressure chemical ionization (APCI) and atmospheric pressure photoionization (APPI) are some of the common variants of the technique. CI mass spectrometry finds general application in the identification, structure elucidation and quantitation of organic compounds as well as some utility in biochemical analysis. Samples to be analyzed must be in vapour form, or else, must be vapourized before introduction into the source.

Electron-capture dissociation (ECD) is a method of fragmenting gas-phase ions for structure elucidation of peptides and proteins in tandem mass spectrometry. It is one of the most widely used techniques for activation and dissociation of mass selected precursor ion in MS/MS. It involves the direct introduction of low-energy electrons to trapped gas-phase ions.
Gas phase ion chemistry is a field of science encompassed within both chemistry and physics. It is the science that studies ions and molecules in the gas phase, most often enabled by some form of mass spectrometry. By far the most important applications for this science is in studying the thermodynamics and kinetics of reactions. For example, one application is in studying the thermodynamics of the solvation of ions. Ions with small solvation spheres of 1, 2, 3... solvent molecules can be studied in the gas phase and then extrapolated to bulk solution.
In mass spectrometry, direct analysis in real time (DART) is an ion source that produces electronically or vibronically excited-state species from gases such as helium, argon, or nitrogen that ionize atmospheric molecules or dopant molecules. The ions generated from atmospheric or dopant molecules undergo ion-molecule reactions with the sample molecules to produce analyte ions. Analytes with low ionization energy may be ionized directly. The DART ionization process can produce positive or negative ions depending on the potential applied to the exit electrode.
Penning ionization is a form of chemi-ionization, an ionization process involving reactions between neutral atoms or molecules. The Penning effect is put to practical use in applications such as gas-discharge neon lamps and fluorescent lamps, where the lamp is filled with a Penning mixture to improve the electrical characteristics of the lamps.
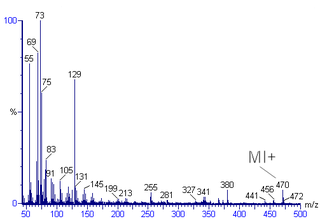
Mass spectral interpretation is the method employed to identify the chemical formula, characteristic fragment patterns and possible fragment ions from the mass spectra. Mass spectra is a plot of relative abundance against mass-to-charge ratio. It is commonly used for the identification of organic compounds from electron ionization mass spectrometry. Organic chemists obtain mass spectra of chemical compounds as part of structure elucidation and the analysis is part of many organic chemistry curricula.

Distonic ions are chemical species that contain ionic charges and radical sites in different locations, unlike regular radicals where the formal charge and unpaired electron are in the same location. These molecular species are created by ionization of either zwitterions or diradicals; ultimately, a neutral molecule loses an electron. Through experimental research distonic radicals have been found to be extremely stable gas phase ions and can be separated into different classes depending on the inherent features of the charged portion of the ion.
Electron capture ionization is the ionization of a gas phase atom or molecule by attachment of an electron to create an ion of the form . The reaction is

In mass spectrometry, fragmentation is the dissociation of energetically unstable molecular ions formed from passing the molecules mass spectrum. These reactions are well documented over the decades and fragmentation patterns are useful to determine the molar weight and structural information of unknown molecules. Fragmentation that occurs in tandem mass spectrometry experiments has been a recent focus of research, because this data helps facilitate the identification of molecules.

Collision-induced dissociation (CID), also known as collisionally activated dissociation (CAD), is a mass spectrometry technique to induce fragmentation of selected ions in the gas phase. The selected ions are usually accelerated by applying an electrical potential to increase the ion kinetic energy and then allowed to collide with neutral molecules. In the collision, some of the kinetic energy is converted into internal energy which results in bond breakage and the fragmentation of the molecular ion into smaller fragments. These fragment ions can then be analyzed by tandem mass spectrometry.
Atmospheric pressure laser ionization is an atmospheric pressure ionization method for mass spectrometry (MS). Laser light in the UV range is used to ionize molecules in a resonance-enhanced multiphoton ionization (REMPI) process. It is a selective and sensitive ionization method for aromatic and polyaromatic compounds. Atmospheric photoionization is the latest in development of atmospheric ionization methods.
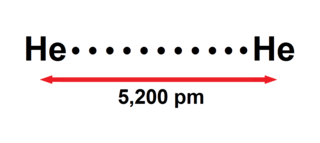
The helium dimer is a van der Waals molecule with formula He2 consisting of two helium atoms. This chemical is the largest diatomic molecule—a molecule consisting of two atoms bonded together. The bond that holds this dimer together is so weak that it will break if the molecule rotates, or vibrates too much. It can only exist at very low cryogenic temperatures.
The magnesium argide ion, MgAr+ is an ion composed of one ionised magnesium atom, Mg+ and an argon atom. It is important in inductively coupled plasma mass spectrometry and in the study of the field around the magnesium ion. The ionization potential of magnesium is lower than the first excitation state of argon, so the positive charge in MgAr+ will reside on the magnesium atom. Neutral MgAr molecules can also exist in an excited state.