
In immunology, an antigen (Ag) is a molecule or molecular structure, such as may be present on the outside of a pathogen, that can be bound by an antigen-specific antibody or B-cell antigen receptor. The presence of antigens in the body normally triggers an immune response. The Ag abbreviation stands for an antibody generator.

An antibody (Ab), also known as an immunoglobulin (Ig), is a large, Y-shaped protein used by the immune system to identify and neutralize foreign objects such as pathogenic bacteria and viruses. The antibody recognizes a unique molecule of the pathogen, called an antigen. Each tip of the "Y" of an antibody contains a paratope that is specific for one particular epitope on an antigen, allowing these two structures to bind together with precision. Using this binding mechanism, an antibody can tag a microbe or an infected cell for attack by other parts of the immune system, or can neutralize it directly.

B cells, also known as B lymphocytes, are a type of white blood cell of the lymphocyte subtype. They function in the humoral immunity component of the adaptive immune system by secreting antibodies. Additionally, B cells present antigens and secrete cytokines. In mammals, B cells mature in the bone marrow, which is at the core of most bones. In birds, B cells mature in the bursa of Fabricius, a lymphoid organ where they were first discovered by Chang and Glick, and not from bone marrow as commonly believed.
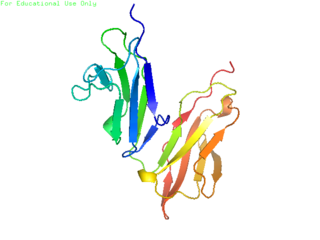
CD32, also known as FcγRII or FCGR2, is a surface receptor glycoprotein belonging to the Ig gene superfamily. CD32 can be found on the surface of a variety of immune cells. CD32 has a low-affinity for the Fc region of IgG antibodies in monomeric form, but high affinity for IgG immune complexes. CD32 has two major functions: cellular response regulation, and the uptake of immune complexes. Cellular responses regulated by CD32 include phagocytosis, cytokine stimulation, and endocytic transport. Dysregulated CD32 is associated with different forms of autoimmunity, including systemic lupus erythematosus. In humans, there are three major CD32 subtypes: CD32A, CD32B, and CD32C. While CD32A and CD32C are involved in activating cellular responses, CD32B is inhibitory.
An immunotoxin is an artificial protein consisting of a targeting portion linked to a toxin. When the protein binds to that cell, it is taken in through endocytosis, and the toxin kills the cell. They are used for the treatment of some kinds of cancer and a few viral infections.

Superantigens (SAgs) are a class of antigens that result in excessive activation of the immune system. Specifically it causes non-specific activation of T-cells resulting in polyclonal T cell activation and massive cytokine release. SAgs are produced by some pathogenic viruses and bacteria most likely as a defense mechanism against the immune system. Compared to a normal antigen-induced T-cell response where 0.0001-0.001% of the body's T-cells are activated, these SAgs are capable of activating up to 20% of the body's T-cells. Furthermore, Anti-CD3 and Anti-CD28 antibodies (CD28-SuperMAB) have also shown to be highly potent superantigens.
Chimeric antigen receptor T cells are T cells that have been genetically engineered to produce an artificial T-cell receptor for use in immunotherapy.
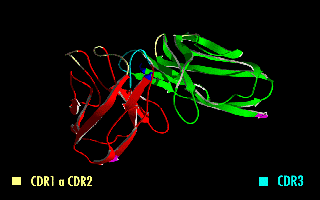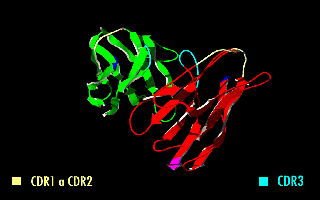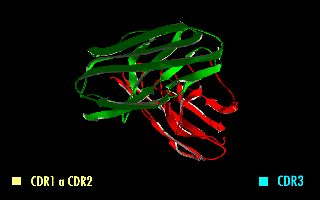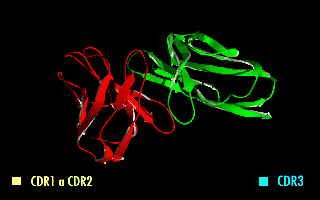
A single-chain variable fragment (scFv) is not actually a fragment of an antibody, but instead is a fusion protein of the variable regions of the heavy (VH) and light chains (VL) of immunoglobulins, connected with a short linker peptide of ten to about 25 amino acids. The linker is usually rich in glycine for flexibility, as well as serine or threonine for solubility, and can either connect the N-terminus of the VH with the C-terminus of the VL, or vice versa. This protein retains the specificity of the original immunoglobulin, despite removal of the constant regions and the introduction of the linker. The image to the right shows how this modification usually leaves the specificity unaltered.

Cancer immunotherapy is the artificial stimulation of the immune system to treat cancer, improving on the immune system's natural ability to fight the disease. It is an application of the fundamental research of cancer immunology and a growing subspeciality of oncology.

The T-cell receptor (TCR) is a protein complex found on the surface of T cells, or T lymphocytes, that is responsible for recognizing fragments of antigen as peptides bound to major histocompatibility complex (MHC) molecules. The binding between TCR and antigen peptides is of relatively low affinity and is degenerate: that is, many TCRs recognize the same antigen peptide and many antigen peptides are recognized by the same TCR.

A Fc receptor is a protein found on the surface of certain cells – including, among others, B lymphocytes, follicular dendritic cells, natural killer cells, macrophages, neutrophils, eosinophils, basophils, human platelets, and mast cells – that contribute to the protective functions of the immune system. Its name is derived from its binding specificity for a part of an antibody known as the Fc region. Fc receptors bind to antibodies that are attached to infected cells or invading pathogens. Their activity stimulates phagocytic or cytotoxic cells to destroy microbes, or infected cells by antibody-mediated phagocytosis or antibody-dependent cell-mediated cytotoxicity. Some viruses such as flaviviruses use Fc receptors to help them infect cells, by a mechanism known as antibody-dependent enhancement of infection.

Monoclonal antibody therapy is a form of immunotherapy that uses monoclonal antibodies (mAb) to bind monospecifically to certain cells or proteins. The objective is that this treatment will stimulate the patient's immune system to attack those cells. Alternatively, in radioimmunotherapy a radioactive dose localizes a target cell line, delivering lethal chemical doses. More recently antibodies have been used to bind to molecules involved in T-cell regulation to remove inhibitory pathways that block T-cell responses. This is known as immune checkpoint therapy.

The fragment crystallizable region is the tail region of an antibody that interacts with cell surface receptors called Fc receptors and some proteins of the complement system. This property allows antibodies to activate the immune system. In IgG, IgA and IgD antibody isotypes, the Fc region is composed of two identical protein fragments, derived from the second and third constant domains of the antibody's two heavy chains; IgM and IgE Fc regions contain three heavy chain constant domains in each polypeptide chain. The Fc regions of IgGs bear a highly conserved N-glycosylation site. Glycosylation of the Fc fragment is essential for Fc receptor-mediated activity. The N-glycans attached to this site are predominantly core-fucosylated diantennary structures of the complex type. In addition, small amounts of these N-glycans also bear bisecting GlcNAc and α-2,6 linked sialic acid residues.
CD16, also known as FcγRIII, is a cluster of differentiation molecule found on the surface of natural killer cells, neutrophils, monocytes, and macrophages. CD16 has been identified as Fc receptors FcγRIIIa (CD16a) and FcγRIIIb (CD16b), which participate in signal transduction. The most well-researched membrane receptor implicated in triggering lysis by NK cells, CD16 is a molecule of the immunoglobulin superfamily (IgSF) involved in antibody-dependent cellular cytotoxicity (ADCC). It can be used to isolate populations of specific immune cells through fluorescent-activated cell sorting (FACS) or magnetic-activated cell sorting, using antibodies directed towards CD16.
A bispecific monoclonal antibody is an artificial protein that can simultaneously bind to two different types of antigen. BsMabs can be manufactured in several structural formats, and current applications have been explored for cancer immunotherapy and drug delivery.

Fc fragment of IgA receptor (FCAR) is a human gene that codes for the transmembrane receptor FcαRI, also known as CD89. FcαRI binds the heavy-chain constant region of Immunoglubulin A (IgA) antibodies. FcαRI is present on the cell surface of myeloid lineage cells, including neutrophils, monocytes, macrophages, and eosinophils, though it is notably absent from intestinal macrophages and does not appear on mast cells. FcαRI plays a role in both pro- and anti-inflammatory responses depending on the state of IgA bound. Inside-out signaling primes FcαRI in order for it to bind its ligand, while outside-in signaling caused by ligand binding depends on FcαRI association with the Fc receptor gamma chain.

A trifunctional antibody is a monoclonal antibody with binding sites for two different antigens, typically CD3 and a tumor antigen, making it a type of bispecific monoclonal antibody. In addition, its intact Fc-part can bind to an Fc receptor on accessory cells like conventional monospecific antibodies. The net effect is that this type of drug links T cells and monocytes/macrophages, natural killer cells, dendritic cells or other Fc receptor expressing cells to the tumor cells, leading to their destruction.

ImmTACs are a class of bispecific biological drug being investigated for the treatment of cancer and viral infections which combines engineered cancer-recognizing TCRs with immune activating complexes. ImmTACs target cancerous or virally infected cells through binding human leukocyte antigen (HLA) presented peptide antigens and redirect the host's cytotoxic T cells to recognise and kill them.
Cytokine-induced killer cells (CIK) cells are a group of immune effector cells featuring a mixed T- and natural killer (NK) cell-like phenotype. They are generated by ex vivo incubation of human peripheral blood mononuclear cells (PBMC) or cord blood mononuclear cells with interferon-gamma (IFN-γ), anti-CD3 antibody, recombinant human interleukin (IL-) 1 and recombinant human interleukin (IL)-2.
Recombinant antibodies are antibody fragments produced by using recombinant antibody coding genes. They mostly consist of a heavy and light chain of the variable region of immunoglobulin. Recombinant antibodies have many advantages in both medical and research applications, which make them a popular subject of exploration and new production against specific targets. The most commonly used form is the single chain variable fragment (scFv), which has shown the most promising traits exploitable in human medicine and research. In contrast to monoclonal antibodies produced by hybridoma technology, which may lose the capacity to produce the desired antibody over time or the antibody may undergo unwanted changes, which affect its functionality, recombinant antibodies produced in phage display maintain high standard of specificity and low immunogenicity.













