
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no other functional groups form a homologous series with the general chemical formula CnH2n−2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature. Like other hydrocarbons, alkynes are generally hydrophobic.

Protein primary structure is the linear sequence of amino acids in a peptide or protein. By convention, the primary structure of a protein is reported starting from the amino-terminal (N) end to the carboxyl-terminal (C) end. Protein biosynthesis is most commonly performed by ribosomes in cells. Peptides can also be synthesized in the laboratory. Protein primary structures can be directly sequenced, or inferred from DNA sequences.
The following outline is provided as an overview of and topical guide to organic chemistry:

Karl Barry Sharpless is an American chemist and Nobel Laureate known for his work on stereoselective reactions and click chemistry.
The 1,3-dipolar cycloaddition is a chemical reaction between a 1,3-dipole and a dipolarophile to form a five-membered ring. The earliest 1,3-dipolar cycloadditions were described in the late 19th century to the early 20th century, following the discovery of 1,3-dipoles. Mechanistic investigation and synthetic application were established in the 1960s, primarily through the work of Rolf Huisgen. Hence, the reaction is sometimes referred to as the Huisgen cycloaddition. 1,3-dipolar cycloaddition is an important route to the regio- and stereoselective synthesis of five-membered heterocycles and their ring-opened acyclic derivatives. The dipolarophile is typically an alkene or alkyne, but can be other pi systems. When the dipolarophile is an alkyne, aromatic rings are generally produced.

Step-growth polymerization refers to a type of polymerization mechanism in which bi-functional or multifunctional monomers react to form first dimers, then trimers, longer oligomers and eventually long chain polymers. Many naturally occurring and some synthetic polymers are produced by step-growth polymerization, e.g. polyesters, polyamides, polyurethanes, etc. Due to the nature of the polymerization mechanism, a high extent of reaction is required to achieve high molecular weight. The easiest way to visualize the mechanism of a step-growth polymerization is a group of people reaching out to hold their hands to form a human chain—each person has two hands. There also is the possibility to have more than two reactive sites on a monomer: In this case branched polymers production take place.
In chemical synthesis, "click" chemistry is a class of biocompatible small molecule reactions commonly used in bioconjugation, allowing the joining of substrates of choice with specific biomolecules. Click chemistry is not a single specific reaction, but describes a way of generating products that follow examples in nature, which also generates substances by joining small modular units. In many applications, click reactions join a biomolecule and a reporter molecule. Click chemistry is not limited to biological conditions: the concept of a "click" reaction has been used in pharmacological and various biomimetic applications. However, they have been made notably useful in the detection, localization and qualification of biomolecules.
The azide-alkyne Huisgen cycloaddition is a 1,3-dipolar cycloaddition between an azide and a terminal or internal alkyne to give a 1,2,3-triazole. Rolf Huisgen was the first to understand the scope of this organic reaction. American chemist Karl Barry Sharpless has referred to this cycloaddition as "the cream of the crop" of click chemistry and "the premier example of a click reaction."
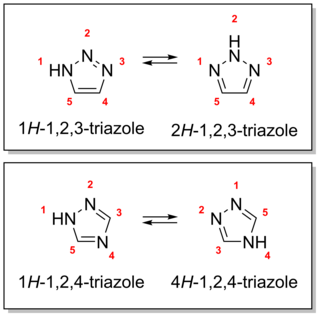
A triazole refers to any of the heterocyclic compounds with molecular formula C2H3N3, having a five-membered ring of two carbon atoms and three nitrogen atoms. There are two sets of isomers that differ in the relative positions of the three nitrogen atoms, namely 1,2,3-Triazoles and 1,2,4-Triazoles. Each of these has two tautomers that differ by which nitrogen has a hydrogen bonded to it.
1,2,3-Triazole is one of a pair of isomeric chemical compounds with molecular formula C2H3N3, called triazoles, which have a five-membered ring of two carbon atoms and three nitrogen atoms. 1,2,3-Triazole is a basic aromatic heterocycle.
In organic chemistry, a cycloalkyne is the cyclic analog of an alkyne. A cycloalkyne consists of a closed ring of carbon atoms containing one or more triple bonds. Cycloalkynes have a general formula CnH2n−4. Because of the linear nature of the C–C≡C–C alkyne unit, cycloalkynes can be highly strained and can only exist when the number of carbon atoms in the ring is great enough to provide the flexibility necessary to accommodate this geometry. Large alkyne-containing carbocycles may be virtually unstrained, while the smallest constituents of this class of molecules may experience so much strain that they have yet to be observed experimentally. Cyclooctyne (C8H12) is the smallest cycloalkyne capable of being isolated and stored as a stable compound. Despite this, smaller cycloalkynes can be produced and trapped through reactions with other organic molecules or through complexation to transition metals.
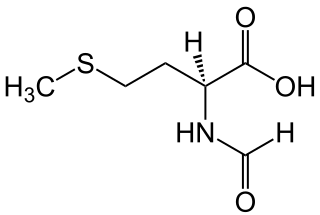
N-Formylmethionine is a derivative of the amino acid methionine in which a formyl group has been added to the amino group. It is specifically used for initiation of protein synthesis from bacterial and organellar genes, and may be removed post-translationally.
Bioconjugation is a chemical strategy to form a stable covalent link between two molecules, at least one of which is a biomolecule.
The Bergmann degradation is a series of chemical reactions designed to remove a single amino acid from the carboxylic acid (C-terminal) end of a peptide. First demonstrated by Max Bergmann in 1934, it is a rarely used method for sequencing peptides. The later developed Edman degradation is an improvement upon the Bergmann degradation, instead cleaving the N-terminal amino acid of peptides to produce a hydantoin containing the desired amino acid.
A dermal patch or skin patch is a medicated adhesive patch that is placed on the skin to deliver a medication into the skin. This is in contrast to a transdermal patch, which delivers the medication through the skin and into the bloodstream.

3-Azidocoumarin is an organic compound that is used in the area of bioconjugation. It is a derivative of coumarin, a natural product and precursor for the widely used Coumadin. Azidocoumarin has emerged as a widely applicable labeling agent in diverse biological systems. In particular, it participates in the aptly named click reaction with alkynes. Bioconjugation involves the labeling of certain cellular components and is applicable to fields such a proteomics and functional genomics with a detachable, fluorescent tag.
The term bioorthogonal chemistry refers to any chemical reaction that can occur inside of living systems without interfering with native biochemical processes. The term was coined by Carolyn R. Bertozzi in 2003. Since its introduction, the concept of the bioorthogonal reaction has enabled the study of biomolecules such as glycans, proteins, and lipids in real time in living systems without cellular toxicity. A number of chemical ligation strategies have been developed that fulfill the requirements of bioorthogonality, including the 1,3-dipolar cycloaddition between azides and cyclooctynes, between nitrones and cyclooctynes, oxime/hydrazone formation from aldehydes and ketones, the tetrazine ligation, the isocyanide-based click reaction, and most recently, the quadricyclane ligation.
Copper-free click chemistry is a bioorthogonal reaction as a variant of an azide alkyne Huisgen cycloaddition. By eliminating cytotoxic copper catalysts, the reaction proceeds without live cell toxicity. It was developed as a faster alternative to the Staudinger ligation, with the first generation producing rate constants over 63 times faster.
PRIME is a molecular biology research tool developed by Alice Y. Ting and the Ting Lab at MIT for site-specific labeling of proteins in living cells with chemical probes. Probes often have useful biophysical properties, such as fluorescence, and allow imaging of proteins. Ultimately, PRIME enables scientists to study functions of specific proteins of interest.
An organic azide is organic compounds containing the azide (N3) functional group. Because of the hazards associated with their use, few azides are used commercially although they exhibit interesting reactivity for researchers. Low molecular weight azides are considered especially hazardous and are avoided. In the research laboratory, azides are precursors to amines. They are also popular for their participation in the "click reaction" and in Staudinger ligation. These two reactions are generally quite reliable, lending themselves to combinatorial chemistry.










