
The mesoderm is the middle layer of the three germ layers that develops during gastrulation in the very early development of the embryo of most animals. The outer layer is the ectoderm, and the inner layer is the endoderm.
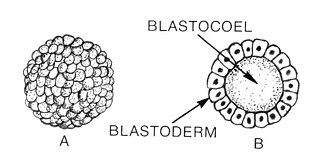
Blastulation is the stage in early animal embryonic development that produces the blastula. In mammalian development the blastula develops into the blastocyst with a differentiated inner cell mass and an outer trophectoderm. The blastula is a hollow sphere of cells known as blastomeres surrounding an inner fluid-filled cavity called the blastocoel. Embryonic development begins with a sperm fertilizing an egg cell to become a zygote, which undergoes many cleavages to develop into a ball of cells called a morula. Only when the blastocoel is formed does the early embryo become a blastula. The blastula precedes the formation of the gastrula in which the germ layers of the embryo form.

Gastrulation is the stage in the early embryonic development of most animals, during which the blastula, or in mammals the blastocyst is reorganized into a multilayered structure known as the gastrula. Before gastrulation, the embryo is a continuous epithelial sheet of cells; by the end of gastrulation, the embryo has begun differentiation to establish distinct cell lineages, set up the basic axes of the body, and internalized one or more cell types including the prospective gut.

The ectoderm is one of the three primary germ layers formed in early embryonic development. It is the outermost layer, and is superficial to the mesoderm and endoderm. It emerges and originates from the outer layer of germ cells. The word ectoderm comes from the Greek ektos meaning "outside", and derma meaning "skin".
Organogenesis is the phase of embryonic development that starts at the end of gastrulation and continues until birth. During organogenesis, the three germ layers formed from gastrulation form the internal organs of the organism.

In developmental biology, animal embryonic development, also known as animal embryogenesis, is the developmental stage of an animal embryo. Embryonic development starts with the fertilization of an egg cell (ovum) by a sperm cell, (spermatozoon). Once fertilized, the ovum becomes a single diploid cell known as a zygote. The zygote undergoes mitotic divisions with no significant growth and cellular differentiation, leading to development of a multicellular embryo after passing through an organizational checkpoint during mid-embryogenesis. In mammals, the term refers chiefly to the early stages of prenatal development, whereas the terms fetus and fetal development describe later stages.

The neural plate is a key developmental structure that serves as the basis for the nervous system. Cranial to the primitive node of the embryonic primitive streak, ectodermal tissue thickens and flattens to become the neural plate. The region anterior to the primitive node can be generally referred to as the neural plate. Cells take on a columnar appearance in the process as they continue to lengthen and narrow. The ends of the neural plate, known as the neural folds, push the ends of the plate up and together, folding into the neural tube, a structure critical to brain and spinal cord development. This process as a whole is termed primary neurulation.
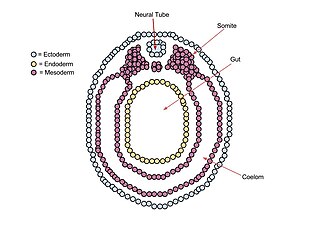
A neurula is a vertebrate embryo at the early stage of development in which neurulation occurs. The neurula stage is preceded by the gastrula stage; consequentially, neurulation is preceded by gastrulation. Neurulation marks the beginning of the process of organogenesis.
The primitive node is the organizer for gastrulation in most amniote embryos. In birds it is known as Hensen's node, and in amphibians it is known as the Spemann-Mangold organizer. It is induced by the Nieuwkoop center in amphibians, or by the posterior marginal zone in amniotes including birds.

In amniote embryonic development, the epiblast is one of two distinct cell layers arising from the inner cell mass in the mammalian blastocyst, or from the blastula in reptiles and birds, the other layer is the hypoblast. It derives the embryo proper through its differentiation into the three primary germ layers, ectoderm, mesoderm and endoderm, during gastrulation. The amnionic ectoderm and extraembryonic mesoderm also originate from the epiblast.
Chordin is a protein with a prominent role in dorsal–ventral patterning during early embryonic development. In humans it is encoded for by the CHRD gene.
In the field of developmental biology, regional differentiation is the process by which different areas are identified in the development of the early embryo. The process by which the cells become specified differs between organisms.

The development of fishes is unique in some specific aspects compared to the development of other animals.
The Nodal signaling pathway is a signal transduction pathway important in regional and cellular differentiation during embryonic development.

In Xenopus laevis, the specification of the three germ layers occurs at the blastula stage. Great efforts have been made to determine the factors that specify the endoderm and mesoderm. On the other hand, only a few examples of genes that are required for ectoderm specification have been described in the last decade. The first molecule identified to be required for the specification of ectoderm was the ubiquitin ligase Ectodermin ; later, it was found that the deubiquitinating enzyme, FAM/USP9x, is able to overcome the effects of ubiquitination made by Ectodermin in Smad4. Two transcription factors have been proposed to control gene expression of ectodermal specific genes: POU91/Oct3/4 and FoxIe1/Xema. A new factor specific for the ectoderm, XFDL156, has shown to be essential for suppression of mesoderm differentiation from pluripotent cells.
This article is about the role of Fibroblast Growth Factor Signaling in Mesoderm Formation.

Vegetal rotation is a morphogenetic movement that drives mesoderm internalization during gastrulation in amphibian embryos. The internalization of vegetal cells prior to gastrulation was first observed in the 1930s by Abraham Mandel Schechtman through the use of vital dye labeling experiments in Triturus torosus embryos. More recently, Winklbauer and Schürfeld (1999) described the internal movements in more detail using pregastrular explants of Xenopus laevis.
Xbra is a homologue of Brachyury (T) gene for Xenopus. It is a transcription activator involved in vertebrate gastrulation which controls posterior mesoderm patterning and notochord differentiation by activating transcription of genes expressed throughout mesoderm. The effects of Xbra is concentration dependent where concentration gradient controls the development of specific types of mesoderm in Xenopus. Xbra results of the expression of the FGF transcription factor, synthesized by the ventral endoderm. So while ventral mesoderm is characterized by a high concentration of FGF and Xbra, the dorsal mesoderm is characterized by a reunion of two others transcription factors, Siamois and XnR, which activates the synthesis of Goosecoid Transcription Factor. Goosecoid enables the depletion of Xbra. In a nutshell, high concentrations of Xbra induce ventral mesoderm while low concentration stimulates the formation of a back.
The Spemann-Mangold organizer is a group of cells that are responsible for the induction of the neural tissues during development in amphibian embryos. First described in 1924 by Hans Spemann and Hilde Mangold, the introduction of the organizer provided evidence that the fate of cells can be influenced by factors from other cell populations. This discovery significantly impacted the world of developmental biology and fundamentally changed the understanding of early development.

The dorsal lip of the blastopore is a structure that forms during early embryonic development and is important for its role in organizing the germ layers. The dorsal lip is formed during early gastrulation as folding of tissue along the involuting marginal zone of the blastocoel forms an opening known as the blastopore. It is particularly important for its role in neural induction through the default model, where signaling from the dorsal lip protects a region of the epiblast from becoming epidermis, thus allowing it to develop to its default neural tissue.












