
Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential, as a measurable and quantitative phenomenon, and identifiable chemical change, with either electrical potential as an outcome of a particular chemical change, or vice versa. These reactions involve electrons moving between electrodes via an electronically-conducting phase, separated by an ionically-conducting and electronically insulating electrolyte.

Electroplating is a general name for processes that create a metal coating on a solid substrate through the reduction of cations of that metal by means of a direct electric current. The part to be coated acts as the cathode of an electrolytic cell; the electrolyte is a solution of a salt of the metal to be coated; and the anode is usually either a block of that metal, or of some inert conductive material. The current is provided by an external power supply.

Corrosion is a natural process that converts a refined metal into a more chemically stable form such as oxide, hydroxide, or sulfide. It is the gradual destruction of materials by chemical and/or electrochemical reaction with their environment. Corrosion engineering is the field dedicated to controlling and preventing corrosion.

A galvanic anode, or sacrificial anode, is the main component of a galvanic cathodic protection (CP) system used to protect buried or submerged metal structures from corrosion.

A lemon battery is a simple battery often made for the purpose of education. Typically, a piece of zinc metal and a piece of copper are inserted into a lemon and connected by wires. Power generated by reaction of the metals is used to power a small device such as a light emitting diode (LED).
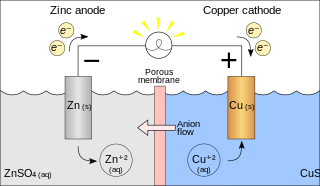
A galvanic cell or voltaic cell, named after the scientists Luigi Galvani and Alessandro Volta, respectively, is an electrochemical cell in which an electric current is generated from spontaneous redox reactions. A common apparatus generally consists of two different metals, each immersed in separate beakers containing their respective metal ions in solution that are connected by a salt bridge.

Brazing is a metal-joining process in which two or more metal items are joined together by melting and flowing a filler metal into the joint, with the filler metal having a lower melting point than the adjoining metal.

An electrolytic cell is an electrochemical cell that uses electrical energy to drive a non-spontaneous redox reaction. It is often used to decompose chemical compounds, in a process called electrolysis—the Greek word lysis means to break up. Important examples of electrolysis are the decomposition of water into hydrogen and oxygen, and bauxite into aluminium and other chemicals. Electroplating is done using an electrolytic cell. Electrolysis is a technique that uses a direct electric current (DC).

Chrome plating is a technique of electroplating a thin layer of chromium onto a metal object. The after product of chrome plating is called chrome. The chromed layer can be decorative, provide corrosion resistance, ease cleaning procedures, or increase surface hardness. Sometimes, a less expensive imitator of chrome may be used for aesthetic purposes.

Anodizing is an electrolytic passivation process used to increase the thickness of the natural oxide layer on the surface of metal parts.
Plating is a surface covering in which a metal is deposited on a conductive surface. Plating has been done for hundreds of years; it is also critical for modern technology. Plating is used to decorate objects, for corrosion inhibition, to improve solderability, to harden, to improve wearability, to reduce friction, to improve paint adhesion, to alter conductivity, to improve IR reflectivity, for radiation shielding, and for other purposes. Jewelry typically uses plating to give a silver or gold finish.

Gold plating is a method of depositing a thin layer of gold onto the surface of another metal, most often copper or silver, by chemical or electrochemical plating. This article covers plating methods used in the modern electronics industry; for more traditional methods, often used for much larger objects, see gilding.

Metallizing is the general name for the technique of coating metal on the surface of objects. Metallic coatings may be decorative, protective or functional.
A coating is a covering that is applied to the surface of an object, usually referred to as the substrate. The purpose of applying the coating may be decorative, functional, or both. The coating itself may be an all-over coating, completely covering the substrate, or it may only cover parts of the substrate. An example of all of these types of coating is a product label on many drinks bottles — one side has an all-over functional coating and the other side has one or more decorative coatings in an appropriate pattern to form the words and images.

Electroless plating, also known as chemical plating or autocatalytic plating, is a class of industrial chemical processes that create metal coatings on various materials by autocatalytic chemical reduction of metal cations in a liquid bath. This class is contrasted with electroplating processes, such as galvanization, where the reduction is achieved by an externally generated electric current.

Electroless nickel-phosphorus plating is a chemical process that deposits an even layer of nickel-phosphorus alloy on the surface of a solid substrate, like metal or plastic. The process involves dipping the substrate in a water solution containing nickel salt and a phosphorus-containing reducing agent, usually a hypophosphite salt. It is the most common version of electroless nickel plating and is often referred just by that name. A similar process uses a borohydride reducing agent, yielding a nickel-boron coating instead.
Electrogalvanizing is a process in which a layer of zinc is bonded to steel in order to protect against corrosion. The process involves electroplating, running a current of electricity through a saline/zinc solution with a zinc anode and steel conductor. Such Zinc electroplating or Zinc alloy electroplating maintains a dominant position among other electroplating process options, based upon electroplated tonnage per annum. According to the International Zinc Association, more than 5 million tons are used yearly for both hot dip galvanizing and electroplating. The plating of zinc was developed at the beginning of the 20th century. At that time, the electrolyte was cyanide based. A significant innovation occurred in the 1960s, with the introduction of the first acid chloride based electrolyte. The 1980s saw a return to alkaline electrolytes, only this time, without the use of cyanide. The most commonly used electrogalvanized cold rolled steel is SECC steel. Compared to hot dip galvanizing, electroplated zinc offers these significant advantages:
Nickel electroplating is a technique of electroplating a thin layer of nickel onto a metal object. The nickel layer can be decorative, provide corrosion resistance, wear resistance, or used to build up worn or undersized parts for salvage purposes.

Galvanic corrosion is an electrochemical process in which one metal corrodes preferentially when it is in electrical contact with another, in the presence of an electrolyte. A similar galvanic reaction is exploited in primary cells to generate a useful electrical voltage to power portable devices.
Electroless copper plating is a chemical process that deposits an even layer of copper on the surface of a solid substrate, like metal or plastic. The process involves dipping the substrate in a water solution containing copper salts and a reducing agent such as formaldehyde.















