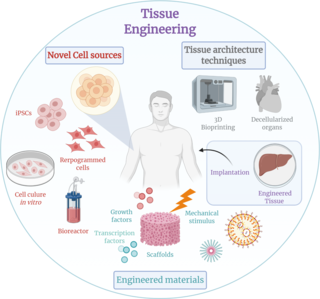
Tissue engineering is a biomedical engineering discipline that uses a combination of cells, engineering, materials methods, and suitable biochemical and physicochemical factors to restore, maintain, improve, or replace different types of biological tissues. Tissue engineering often involves the use of cells placed on tissue scaffolds in the formation of new viable tissue for a medical purpose, but is not limited to applications involving cells and tissue scaffolds. While it was once categorized as a sub-field of biomaterials, having grown in scope and importance, it can is considered as a field of its own.

An artificial heart valve is a one-way valve implanted into a person's heart to replace a heart valve that is not functioning properly. Artificial heart valves can be separated into three broad classes: mechanical heart valves, bioprosthetic tissue valves and engineered tissue valves.
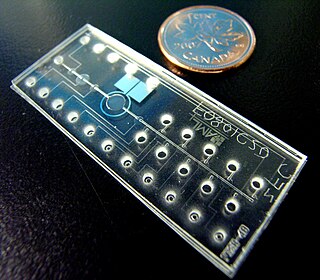
Bio-MEMS is an abbreviation for biomedical microelectromechanical systems. Bio-MEMS have considerable overlap, and is sometimes considered synonymous, with lab-on-a-chip (LOC) and micro total analysis systems (μTAS). Bio-MEMS is typically more focused on mechanical parts and microfabrication technologies made suitable for biological applications. On the other hand, lab-on-a-chip is concerned with miniaturization and integration of laboratory processes and experiments into single chips. In this definition, lab-on-a-chip devices do not strictly have biological applications, although most do or are amenable to be adapted for biological purposes. Similarly, micro total analysis systems may not have biological applications in mind, and are usually dedicated to chemical analysis. A broad definition for bio-MEMS can be used to refer to the science and technology of operating at the microscale for biological and biomedical applications, which may or may not include any electronic or mechanical functions. The interdisciplinary nature of bio-MEMS combines material sciences, clinical sciences, medicine, surgery, electrical engineering, mechanical engineering, optical engineering, chemical engineering, and biomedical engineering. Some of its major applications include genomics, proteomics, molecular diagnostics, point-of-care diagnostics, tissue engineering, single cell analysis and implantable microdevices.
A fibrin scaffold is a network of protein that holds together and supports a variety of living tissues. It is produced naturally by the body after injury, but also can be engineered as a tissue substitute to speed healing. The scaffold consists of naturally occurring biomaterials composed of a cross-linked fibrin network and has a broad use in biomedical applications.
Mechanobiology is an emerging field of science at the interface of biology, engineering, chemistry and physics. It focuses on how physical forces and changes in the mechanical properties of cells and tissues contribute to development, cell differentiation, physiology, and disease. Mechanical forces are experienced and may be interpreted to give biological responses in cells. The movement of joints, compressive loads on the cartilage and bone during exercise, and shear pressure on the blood vessel during blood circulation are all examples of mechanical forces in human tissues. A major challenge in the field is understanding mechanotransduction—the molecular mechanisms by which cells sense and respond to mechanical signals. While medicine has typically looked for the genetic and biochemical basis of disease, advances in mechanobiology suggest that changes in cell mechanics, extracellular matrix structure, or mechanotransduction may contribute to the development of many diseases, including atherosclerosis, fibrosis, asthma, osteoporosis, heart failure, and cancer. There is also a strong mechanical basis for many generalized medical disabilities, such as lower back pain, foot and postural injury, deformity, and irritable bowel syndrome.
An organ-on-a-chip (OOC) is a multi-channel 3-D microfluidic cell culture, integrated circuit (chip) that simulates the activities, mechanics and physiological response of an entire organ or an organ system. It constitutes the subject matter of significant biomedical engineering research, more precisely in bio-MEMS. The convergence of labs-on-chips (LOCs) and cell biology has permitted the study of human physiology in an organ-specific context. By acting as a more sophisticated in vitro approximation of complex tissues than standard cell culture, they provide the potential as an alternative to animal models for drug development and toxin testing.
Neural crest cells are multipotent cells required for the development of cells, tissues and organ systems. A subpopulation of neural crest cells are the cardiac neural crest complex. This complex refers to the cells found amongst the midotic placode and somite 3 destined to undergo epithelial-mesenchymal transformation and migration to the heart via pharyngeal arches 3, 4 and 6.
A bioartificial heart is an engineered heart that contains the extracellular structure of a decellularized heart and cellular components from a different source. Such hearts are of particular interest for therapy as well as research into heart disease. The first bioartificial hearts were created in 2008 using cadaveric rat hearts. In 2014, human-sized bioartificial pig hearts were constructed. Bioartificial hearts have not been developed yet for clinical use, although the recellularization of porcine hearts with human cells opens the door to xenotransplantation.
Regeneration in humans is the regrowth of lost tissues or organs in response to injury. This is in contrast to wound healing, or partial regeneration, which involves closing up the injury site with some gradation of scar tissue. Some tissues such as skin, the vas deferens, and large organs including the liver can regrow quite readily, while others have been thought to have little or no capacity for regeneration following an injury.
Molly S. Shoichet, is a Canadian science professor, specializing in chemistry, biomaterials and biomedical engineering. She was Ontario's first Chief Scientist. Shoichet is a biomedical engineer known for her work in tissue engineering, and is the only person to be a fellow of the three National Academies in Canada.

Eugenia Eduardovna Kumacheva is a University Professor and Distinguished Professor of Chemistry at the University of Toronto. Her research interests span across the fields of fundamental and applied polymers science, nanotechnology, microfluidics, and interface chemistry. She was awarded the L'Oréal-UNESCO Awards for Women in Science in 2008 "for the design and development of new materials with many applications including targeted drug delivery for cancer treatments and materials for high density optical data storage". In 2011, she published a book on the Microfluidic Reactors for Polymer Particles co-authored with Piotr Garstecki. She is Canadian Research Chair in Advanced Polymer Materials. She is Fellow of the Royal Society (FRS) and a Fellow of the Royal Society of Canada (FRSC).

Joyce Y. Wong is an American engineer who is Professor of Biomedical Engineering and Materials Science and Engineering at Boston University. Her research develops novel biomaterials for the early detection treatment of disease. Wong is the Inaugural Director of the Provost's Initiative to promote gender equality and inclusion in STEM at all levels: Advance, Recruit, Retain and Organize Women in STEM. She is a Fellow of the American Association for the Advancement of Science, American Institute for Medical and Biological Engineering and Biomedical Engineering Society.
Tissue engineered heart valves (TEHV) offer a new and advancing proposed treatment of creating a living heart valve for people who are in need of either a full or partial heart valve replacement. Currently, there are over a quarter of a million prosthetic heart valves implanted annually, and the number of patients requiring replacement surgeries is only suspected to rise and even triple over the next fifty years. While current treatments offered such as mechanical valves or biological valves are not deleterious to one's health, they both have their own limitations in that mechanical valves necessitate the lifelong use of anticoagulants while biological valves are susceptible to structural degradation and reoperation. Thus, in situ (in its original position or place) tissue engineering of heart valves serves as a novel approach that explores the use creating a living heart valve composed of the host's own cells that is capable of growing, adapting, and interacting within the human body's biological system.

Christopher S. Chen, born in 1968, is an American biological engineer. He is the William Fairfield Warren Distinguished Professor of Biomedical Engineering at Boston University and member of the Wyss Institute for Biologically Inspired Engineering at Harvard University in Boston.

Milica Radisic is a Serbian Canadian tissue engineer, academic and researcher. She is a professor at the University of Toronto’s Institute of Biomaterials and Biomedical Engineering, and the Department of Chemical Engineering and Applied Chemistry. She co-founded TARA Biosystems and is a senior scientist at the Toronto General Hospital Research Institute.
Katja Schenke-Layland is the Professor of Medical Technologies and Regenerative Medicine, Institute of Biomedical Engineering, Department for Medical Technologies and Regenerative Medicine at the University of Tübingen. She is the Director of the NMI Natural and Medical Sciences Institute at the University Tübingen in Reutlingen, Study Dean of Medical Technologies at the University of Tübingen, and Founding Director of the Institute of Biomedical Engineering at the Medical Faculty of the University Tübingen. She is also the Founding Director of the 3R Center for In Vitro Models and Alternatives to Animal Testing Tübingen.
Shelly R. Peyton is an American chemical engineer who is the Armstrong Professional Development Professor in the Department of CHemical Engineering at the University of Massachusetts Amherst. Her research considers the development of biomaterials to investigate metastatic cancer and potential new therapies.
Intestines-on-a-chip are microfluidic bioengineered 3D-models of the real organ, which better mimic physiological features than conventional 3D intestinal organoid culture. A variety of different intestine-on-a-chip models systems have been developed and refined, all holding their individual strengths and weaknesses and collectively holding great promise to the ultimate goal of establishing these systems as reliable high-throughput platforms for drug testing and personalised medicine. The intestine is a highly complex organ system performing a diverse set of vital tasks, from nutrient digestion and absorption, hormone secretion, and immunological processes to neuronal activity, which makes it particularly challenging to model in vitro.
Amy Rowat is an Associate Professor of biophysics at the University of California in Los Angeles (UCLA) and the first Marcie H. Rothman Presidential Chair in Food Studies. Her scientific research focuses on understanding the physical and mechanical properties of cells in diseases such as cancer. She also organizes public events on the science of cooking.
Aaron R. Wheeler is a Canadian chemist who is a professor of chemistry and biomedical engineering at the University of Toronto since 2005 with cross-appointment at Institute of Biomedical Engineering and Terrence Donnelly Centre for Cellular and Biomolecular Research. His academic laboratory is located at Lash Miller Chemical Laboratories and Terrence Donnelly Centre for Cellular and Biomolecular Research at the University of Toronto. In 2005, Wheeler was appointed as assistant professor and Tier II Canada Research Chair then promoted to associate professor in 2010, full professor in 2013, and in 2018 he became the Tier I Canada Research Chair in Microfluidic Bioanalysis.






