
Mouthwash, mouth rinse, oral rinse, or mouth bath is a liquid which is held in the mouth passively or swirled around the mouth by contraction of the perioral muscles and/or movement of the head, and may be gargled, where the head is tilted back and the liquid bubbled at the back of the mouth.

Myrrh is a gum-resin extracted from a few small, thorny tree species of the Commiphora genus, belonging to the Burseraceae family. Myrrh resin has been used throughout history in medicine, perfumery, and incenses. Myrrh mixed with posca or wine was widely used in many ancient cultures to produce pleasurable feelings and as an anti-inflammatory and analgesic.
Thymol, C10H14O, is a natural monoterpenoid phenol derivative of p-Cymene, isomeric with carvacrol. It occurs naturally in the oil of thyme, and it is extracted from Thymus vulgaris, ajwain, and various other plants as a white crystalline substance of a pleasant aromatic odor and strong antiseptic properties. Thymol also provides the distinctive, strong flavor of the culinary herb thyme, also produced from T. vulgaris. Thymol is only slightly soluble in water at neutral pH, but it is extremely soluble in alcohols and other organic solvents. It is also soluble in strongly alkaline aqueous solutions due to deprotonation of the phenol. Its dissociation constant (pKa) is 10.59±0.10. Thymol absorbs maximum UV radiation at 274 nm.

A lipoxin (LX or Lx), an acronym for lipoxygenase interaction product, is a bioactive autacoid metabolite of arachidonic acid made by various cell types. They are categorized as nonclassic eicosanoids and members of the specialized pro-resolving mediators (SPMs) family of polyunsaturated fatty acid (PUFA) metabolites. Like other SPMs, LXs form during, and then act to resolve, inflammatory responses. Initially, two lipoxins were identified, lipoxin A4 (LXA4) and LXB4, but more recent studies have identified epimers of these two LXs: the epi-lipoxins, 15-epi-LXA4 and 15-epi-LXB4 respectively.
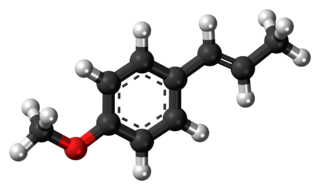
Anethole is an organic compound that is widely used as a flavoring substance. It is a derivative of the aromatic compound allylbenzene and occurs widely in the essential oils of plants. It is in the class of phenylpropanoid organic compounds. It contributes a large component of the odor and flavor of anise and fennel, anise myrtle (Myrtaceae), liquorice (Fabaceae), magnolia blossoms, and star anise (Schisandraceae). Closely related to anethole is its isomer estragole, which is abundant in tarragon (Asteraceae) and basil (Lamiaceae), and has a flavor reminiscent of anise. It is a colorless, fragrant, mildly volatile liquid. Anethole is only slightly soluble in water but exhibits high solubility in ethanol. This trait causes certain anise-flavored liqueurs to become opaque when diluted with water; this is called the ouzo effect.

Tigecycline, sold under the brand name Tygacil, is a tetracycline antibiotic medication for a number of bacterial infections. It is a glycylcycline class drug that is administered intravenously. It was developed in response to the growing rate of antibiotic resistant bacteria such as Staphylococcus aureus, Acinetobacter baumannii, and E. coli. As a tetracycline derivative antibiotic, its structural modifications has expanded its therapeutic activity to include Gram-positive and Gram-negative organisms, including those of multi-drug resistance.
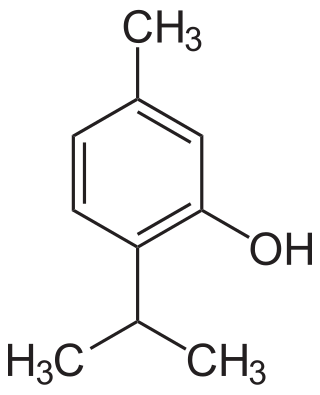
Carvacrol, or cymophenol, C6H3(CH3)(OH)C3H7, is a monoterpenoid phenol. It has a characteristic pungent, warm odor of oregano.

Backhousia citriodora, commonly known as lemon myrtle, lemon scented myrtle or lemon scented ironwood, is a flowering plant in the family Myrtaceae. It is native to the subtropical rainforests of central and south-eastern Queensland, Australia, with a natural distribution from Mackay to Brisbane.
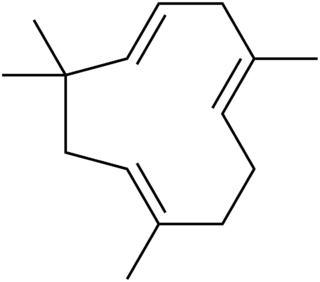
Humulene, also known as α-humulene or α-caryophyllene, is a naturally occurring monocyclic sesquiterpene (C15H24), containing an 11-membered ring and consisting of 3 isoprene units containing three nonconjugated C=C double bonds, two of them being triply substituted and one being doubly substituted. It was first found in the essential oils of Humulus lupulus (hops), from which it derives its name. Humulene is an isomer of β-caryophyllene, and the two are often found together as a mixture in many aromatic plants.
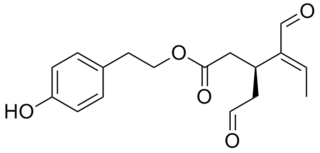
Oleocanthal is a phenylethanoid, or a type of natural phenolic compound found in extra-virgin olive oil. It appears to be responsible for the burning sensation that occurs in the back of the throat when consuming such oil. Oleocanthal is a tyrosol ester and its chemical structure is related to oleuropein, also found in olive oil.

Cymbopogon citratus, commonly known as West Indian lemon grass or simply lemon grass, is a tropical plant native to South Asia and Maritime Southeast Asia and introduced to many tropical regions.

N-formyl peptide receptor 2 (FPR2) is a G-protein coupled receptor (GPCR) located on the surface of many cell types of various animal species. The human receptor protein is encoded by the FPR2 gene and is activated to regulate cell function by binding any one of a wide variety of ligands including not only certain N-Formylmethionine-containing oligopeptides such as N-Formylmethionine-leucyl-phenylalanine (FMLP) but also the polyunsaturated fatty acid metabolite of arachidonic acid, lipoxin A4 (LXA4). Because of its interaction with lipoxin A4, FPR2 is also commonly named the ALX/FPR2 or just ALX receptor.

Protocatechuic acid (PCA) is a dihydroxybenzoic acid, a type of phenolic acid. It is a major metabolite of antioxidant polyphenols found in green tea. It has mixed effects on normal and cancer cells in in vitro and in vivo studies. It is produced commercially from vanillin.
Octenidine dihydrochloride is a cationic surfactant, with a gemini-surfactant structure, derived from 4-aminopyridine. It is active against Gram-positive and Gram-negative bacteria. Since 1987, it has been used primarily in Europe as an antiseptic prior to medical procedures, including on neonates.
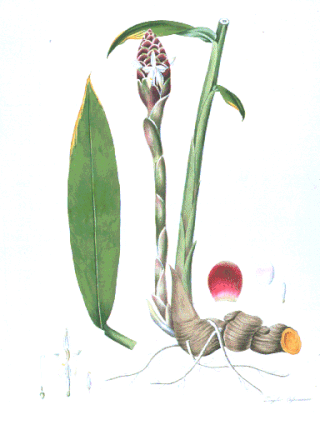
Cassumunar ginger: Zingiber cassumunar, now thought to be a synonym of Zingiber montanum (J.König) Link ex A.Dietr., is a species of plant in the ginger family and is also a relative of galangal. It is called plai (ไพล) in Thailand, in addition to in Isan language and in northern Thai language. The rhizome of variant 'Roxburgh' is used medicinally in massage and even in food in Thailand, and somewhat resembles ginger root or galangal. In aromatherapy, plai oil is used as an essential oil and is believed to ease pain and inflammation. It is also known as ponlei (ពន្លៃ) in Cambodia.
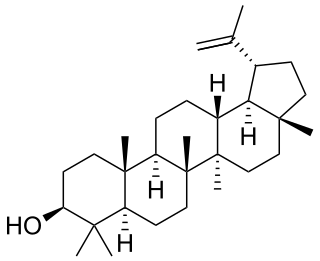
Lupeol is a pharmacologically active pentacyclic triterpenoid. It has several potential medicinal properties, like anticancer and anti-inflammatory activity.
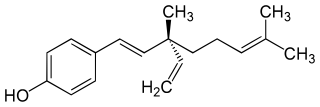
Bakuchiol is a meroterpenoid in the class terpenophenol.

Nimbin is a triterpenoid isolated from the neem tree. Nimbin is thought to be responsible for much of the biological activities of neem oil, and is reported to have anti-inflammatory, antipyretic, fungicidal, antihistamine and antiseptic properties. The neem tree is found in multiple Asian countries such as China, Thailand, and India. Nimbin is part of the chemical family of limonoids and triterpenoids. Nimbin was first extracted in 1942 from neem seeds by Siddiqi et al. Its molecular formula was established by mass-spectrometry along with salannin, a compound whose chemical formula and properties are very close those of nimbin. Nimbin can be extracted from different parts of the neem tree with a solvent or supercritical carbon dioxide. Nimbin is used for different purposes because it has multiple properties such as insecticide, antiviral, antimicrobial, anti-inflammatory, and anti-fungal. Nimbin was commonly used in traditional Indian and Chinese medicine. For example, it can be used to treat skin conditions like eczema and psoriasis.

Dr. Bibi Ameenah Firdaus Gurib-FakimGCSK is a Mauritian politician and biodiversity scientist who served as the sixth president of Mauritius from 2015 to 2018. In December 2014, she was selected to be the presidential candidate of the Alliance Lepep. After Kailash Purryag resigned on 29 May 2015, both Prime Minister Sir Anerood Jugnauth and Leader of the Opposition Paul Berenger positively welcomed her nomination, which was unanimously approved in a vote in the National Assembly.

Interleukin 17F (IL-17F) is signaling protein that is in human is encoded by the IL17F gene and is considered a pro-inflammatory cytokine. This protein belongs to the interleukin 17 family and is mainly produced by the T helper 17 cells after their stimulation with interleukin 23. However, IL-17F can be also produced by a wide range of cell types, including innate immune cells and epithelial cells.


















